 Полная версия
Полная версияOn the various forces of nature and their relations to each other
There is another remarkable circumstance in chemical affinity, which is, that it is capable of either waiting or acting at once. And this is very singular, because we know of nothing of the kind in the forces either of gravitation or cohesion. For instance, here are some oxygen particles, and here is a lump of carbon particles. I am going to put the carbon particles into the oxygen; they can act, but they do not – they are just like this unlighted candle. It stands here quietly on the table, waiting until we want to light it. But it is not so in this other case. Here is a substance, gaseous like the oxygen, and if I put these particles of metal into it, the two combine at once. The copper and the chlorine unite by their power of chemical affinity, and produce a body entirely unlike either of the substances used. And in this other case, it is not that there is any deficiency of affinity between the carbon and oxygen; for the moment I choose to put them in a condition to exert their affinity, you will see the difference. [The piece of charcoal was ignited, and introduced into the jar of oxygen, when the combustion proceeded with vivid scintillations.]
Now, this chemical action is set going exactly as it would be if I had lighted the candle, or as it is when the servant puts coals on and lights the fire: the substances wait until we do something which is able to start the action. Can anything be more beautiful than this combustion of charcoal in oxygen? You must understand that each of these little sparks is a portion of the charcoal, or the bark of the charcoal, thrown off white-hot into the oxygen, and burning in it most brilliantly, as you see. And now let me tell you another thing, or you will go away with a very imperfect notion of the powers and effects of this affinity. There you see some charcoal burning in oxygen. Well, a piece of lead will burn in oxygen just as well as the charcoal does, or indeed better; for absolutely that piece of lead will act at once upon the oxygen as the copper did in the other vessel with regard to the chlorine. And here also a piece of iron: if I light it and put it into the oxygen, it will burn away just as the carbon did. And I will take some lead, and shew you that it will burn in the common atmospheric oxygen at the ordinary temperature. These are the lumps of lead which, you remember, we had the other day – the two pieces which clung together. Now these pieces, if I take them to-day and press them together, will not stick; and the reason is, that they have attracted from the atmosphere a part of the oxygen there present, and have become coated as with a varnish by the oxide of lead, which is formed on the surface by a real process of combustion or combination. There you see the iron burning very well in oxygen; and I will tell you the reason why those scissors and that lead do not take fire whilst they are lying on the table. Here the lead is in a lump, and the coating of oxide remains on its surface; whilst there you see the melted oxide is clearing itself off from the iron, and allowing more and more to go on burning. In this case, however [holding up a small glass tube containing lead pyrophorus.21, the lead has been very carefully produced in fine powder, and put into a glass tube, and hermetically sealed, so as to preserve it; and I expect you will see it take fire at once. This has been made about a month ago, and has thus had time enough to sink down to its normal temperature. What you see, therefore, is the result of chemical affinity alone. [The tube was broken at the end, and the lead poured out on to a piece of paper, whereupon it immediately took fire.] Look, look at the lead burning; why, it has set fire to the paper! Now, that is nothing more than the common affinity always existing between very clean lead and the atmospheric oxygen; and the reason why this iron does not burn until it is made red-hot is, because it has got a coating of oxide about it, which stops the action of the oxygen – putting a varnish, as it were, upon its surface, as we varnish a picture, absolutely forming a substance which prevents the natural chemical affinity between the bodies from acting.
I must now take you a little further in this kind of illustration – or consideration, I would rather call it – of chemical affinity. This attraction between different particles exists also most curiously in cases where they are previously combined with other substances. Here is a little chlorate of potash, containing the oxygen which we found yesterday could be procured from it. It contains the oxygen there combined and held down by its chemical affinity with other things; but still it can combine with sugar, as you saw. This affinity can thus act across substances; and I want you to see how curiously what we call combustion acts with respect to this force of chemical affinity. If I take a piece of phosphorus and set fire to it, and then place a jar of air over the phosphorus, you see the combustion which we are having there on account of chemical affinity (combustion being in all cases the result of chemical affinity). The phosphorus is escaping in that vapour, which will condense into a snow-like mass at the close of the lecture. But suppose I limit the atmosphere, what then? why, even the phosphorus will go out. Here is a piece of camphor, which will burn very well in the atmosphere, and even on water it will float about and burn, by reason of some of its particles gaining access to the air. But if I limit the quantity of air by placing a jar over it, as I am now doing, you will soon find the camphor will go out. Well, why does it go out? Not for want of air, for there is plenty of air remaining in the jar. Perhaps you will be shrewd enough to say, for want of oxygen.
This, therefore, leads us to the inquiry as to whether oxygen can do more than a certain amount of work. The oxygen there (fig. 30) cannot go on burning an unlimited quantity of candle, for that has gone out, as you see; and its amount of chemical attraction or affinity is just as strikingly limited: it can no more be fallen short of or exceeded than can the attraction of gravitation. You might as soon attempt to destroy gravitation, or weight, or all things that exist, as to destroy the exact amount of force exerted by this oxygen. And when I pointed out to you that 8 by weight of oxygen to 1 by weight of hydrogen went to form water, I meant this, that neither of them would combine in different proportions with the other; for you cannot get 10 of hydrogen to combine with 6 of oxygen, or 10 of oxygen to combine with 6 of hydrogen – it must be 8 of oxygen and 1 of hydrogen. Now, suppose I limit the action in this way: this piece of cotton wool burns, as you see, very well in the atmosphere; and I have known of cases of cotton-mills being fired as if with gunpowder, through the very finely-divided particles of cotton being diffused through the atmosphere in the mill, when it has sometimes happened that a flame has caught these raised particles, and it has run from one end of the mill to the other, and blown it up. That, then, is on account of the affinity which the cotton has for the oxygen; but suppose I set fire to this piece of cotton, which is rolled up tightly, it does not go on burning, because I have limited the supply of oxygen, and the inside is prevented from having access to the oxygen, just as it was in the case of the lead by the oxide. But here is some cotton which has been imbued with oxygen in a certain manner. I need not trouble you now with the way it is prepared; it is called gun-cotton.22 See how that burns [setting fire to a piece]; it is very different from the other, because the oxygen that must be present in its proper amount is put there beforehand. And I have here some pieces of paper which are prepared like the gun-cotton23, and imbued with bodies containing oxygen. Here is some which has been soaked in nitrate of strontia – you will see the beautiful red colour of its flame; and here is another which I think contains baryta, which gives that fine green light; and I have here some more which has been soaked in nitrate of copper – it does not burn quite so brightly, but still very beautifully. In all these cases the combustion goes on independent of the oxygen of the atmosphere. And here we have some gunpowder put into a case, in order to shew that it is capable of burning under water. You know that we put it into a gun, shutting off the atmosphere, with shot, and yet the oxygen which it contains supplies the particles with that without which chemical action could not proceed. Now, I have a vessel of water here, and am going to make the experiment of putting this fuse under the water, and you will see whether that water can extinguish it. Here it is burning out of the water, and there it is burning under the water; and so it will continue until exhausted, and all by reason of the requisite amount of oxygen being contained within the substance. It is by this kind of attraction of the different particles one to the other that we are enabled to trace the laws of chemical affinity, and the wonderful variety of the exertions of these laws.
Now, I want you to observe that one great exertion of this power, which is known as chemical affinity, is to produce HEAT and light. You know, as a matter of fact, no doubt, that when bodies burn they give out heat; but it is a curious thing that this heat does not continue – the heat goes away as soon as the action stops, and you see thereby that it depends upon the action during the time it is going on. It is not so with gravitation: this force is continuous, and is just as effective in making that lead press on the table as it was when it first fell there. Nothing occurs there which disappears when the action of falling is over; the pressure is upon the table, and will remain there until the lead is removed; whereas, in the action of chemical affinity to give light and heat, they go away immediately the action is over. This lamp seems to evolve heat and light continuously; but it is owing to a constant stream of air coming into it on all sides, and this work of producing light and heat by chemical affinity will subside as soon as the stream of air is interrupted. What, then, is this curious condition of heat? Why is the evolution of another power of matter, of a power new to us, and which we must consider as if it were now for the very first time brought under our notice? What is heat? We recognise heat by its power of liquefying solid bodies and vaporising liquid bodies, by its power of setting in action, and very often overcoming, chemical affinity. Then, how do we obtain heat? We obtain it in various ways – most abundantly by means of the chemical affinity we have just before been speaking about; but we can also obtain it in many other ways. Friction will produce heat. The Indians rub pieces of wood together until they make them hot enough to take fire; and such things have been known as two branches of a tree rubbing together so hard as to set the tree on fire. I do not suppose I shall set these two pieces of wood on fire by friction; but I can readily produce heat enough to ignite some phosphorus. [The Lecturer here rubbed two pieces of cedar-wood strongly against each other for a minute, and then placed on them a piece of phosphorus, which immediately took fire.] And if you take a smooth metal button stuck on a cork, and rub it on a piece of soft deal wood, you will make it so hot as to scorch wood and paper, and burn a match.
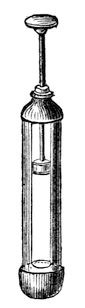
Fig. 31.
I am now going to shew you that we can obtain heat, not by chemical affinity alone, but by the pressure of air. Suppose I take a pellet of cotton and moisten it with a little ether, and put it into a glass tube (fig. 31), and then take a piston and press it down suddenly, I expect I shall be able to burn a little of that ether in the vessel. It wants a suddenness of pressure, or we shall not do what we require. [The piston was forcibly pressed down, when a flame, due to the combustion of the ether, was visible in the lower part of the syringe.] All we want is to get a little ether in vapour, and give fresh air each time, and so we may go on again and again getting heat enough by the compression of air to fire the ether-vapour.
This, then, I think, will be sufficient, accompanied with all you have previously seen, to shew you how we procure heat. And now for the effects of this power. We need not consider many of them on the present occasion, because when you have seen its power of changing ice into water and water into steam, you have seen the two principal results of the application of heat. I want you now to see how it expands all bodies – all bodies but one, and that under limited circumstances. Mr. Anderson will hold a lamp under that retort, and you will see the moment he does so that the air will issue abundantly from the neck, which is under water, because the heat which he applies to the air causes it to expand. And here is a brass rod (fig. 32) which goes through that hole, and fits also accurately into this gauge; but if I make it warm with this spirit-lamp, it will only go in the gauge or through the hole with difficulty; and if I were to put it into boiling-water, it would not go through at all. Again, as soon as the heat escapes from bodies they collapse. See how the air is contracting in the vessel, now that Mr. Anderson has taken away his lamp: the stem of it is filling with water. Notice, too, now, that although I cannot get the tube through this hole or into the gauge, the moment I cool it by dipping it into water, it goes through with perfect facility; so that we have a perfect proof of this power of heat to contract and expand bodies.
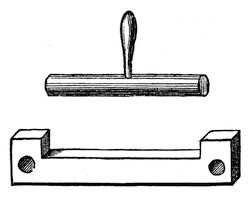
Fig. 32.
LECTURE V.
MAGNETISM – ELECTRICITY
I wonder whether we shall be too deep to-day or not. Remember that we spoke of the attraction by gravitation of all bodies to all bodies by their simple approach. Remember that we spoke of the attraction of particles of the same kind to each other, – that power which keeps them together in masses, – iron attracted to iron, brass to brass, or water to water. Remember that we found, on looking into water, that there were particles of two different kinds attracted to each other; and this was a great step beyond the first simple attraction of gravitation; because here we deal with attraction between different kinds of matter. The hydrogen could attract the oxygen, and reduce it to water, but it could not attract any of its own particles; so that there we obtained a first indication of the existence of two attractions.
To-day we come to a kind of attraction even more curious than the last, namely, the attraction which we find to be of a double nature – of a curious and dual nature. And I want first of all to make the nature of this doubleness clear to you. Bodies are sometimes endowed with a wonderful attraction, which is not found in them in their ordinary state. For instance, here is a piece of shell-lac, having the attraction of gravitation, having the attraction of cohesion; and if I set fire to it, it would have the attraction of chemical affinity to the oxygen in the atmosphere. Now, all these powers we find in it as if they were parts of its substance; but there is another property which I will try and make evident by means of this ball, this bubble of air [a light india-rubber ball, inflated and suspended by a thread]. There is no attraction between this ball and this shell-lac at present: there may be a little wind in the room slightly moving the ball about, but there is no attraction. But if I rub the shell-lac with a piece of flannel [rubbing the shell-lac, and then holding it near the ball], look at the attraction which has arisen out of the shell-lac, simply by this friction, and which I may take away as easily by drawing it gently through my hand. [The Lecturer repeated the experiment of exciting the shell-lac, and then removing the attractive power by drawing it through his hand.] Again, you will see I can repeat this experiment with another substance; for if I take a glass rod and rub it with a piece of silk covered with what we call amalgam, look at the attraction which it has, how it draws the ball towards it; and then, as before, by quietly rubbing it through the hand, the attraction will be all removed again, to come back by friction with this silk.
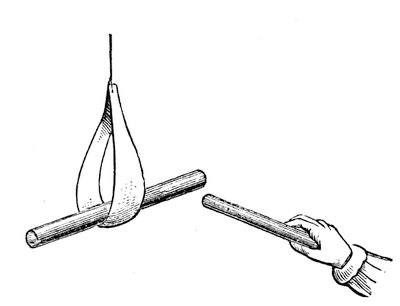
Fig. 33.
But now we come to another fact. I will take this piece of shell-lac and make it attractive by friction; and remember that whenever we get an attraction of gravity, chemical affinity, adhesion, or electricity (as in this case), the body which attracts is attracted also; and just as much as that ball was attracted by the shell-lac, the shell-lac was attracted by the ball. Now, I will suspend this piece of excited shell-lac in a little paper stirrup, in this way (fig. 33), in order to make it move easily, and I will take another piece of shell-lac, and after rubbing it with flannel, will bring them near together. You will think that they ought to attract each other; but now what happens? It does not attract; on the contrary, it very strongly repels, and I can thus drive it round to any extent. These, therefore, repel each other, although they are so strongly attractive – repel each other to the extent of driving this heavy piece of shell-lac round and round in this way. But if I excite this piece of shell-lac, as before, and take this piece of glass and rub it with silk, and then bring them near, what think you will happen? [The Lecturer held the excited glass near the excited shell-lac, when they attracted each other strongly.] You see, therefore, what a difference there is between these two attractions, – they are actually two kinds of attraction concerned in this case, quite different to anything we have met with before; but the force is the same. We have here, then, a double attraction – a dual attraction or force – one attracting, and the other repelling.
Again, to shew you another experiment which will help to make this clear to you. Suppose I set up this rough indicator again [the excited shell-lac suspended in the stirrup] – it is rough, but delicate enough for my purpose; and suppose I take this other piece of shell-lac, and take away the power, which I can do by drawing it gently through the hand; and suppose I take a piece of flannel (fig. 34), which I have shaped into a cap for it and made dry. I will put this shell-lac into the flannel, and here comes out a very beautiful result. I will rub this shell-lac and the flannel together (which I can do by twisting the shell-lac round), and leave them in contact; and then, if I ask, by bringing them nearer our indicator, what is the attractive force? – it is nothing! But if I take them apart, and then ask what will they do when they are separated – why, the shell-lac is strongly repelled, as it was before, but the cap is strongly attractive; and yet if I bring them both together again, there is no attraction – it has all disappeared [the experiment was repeated]. Those two bodies, therefore, still contain this attractive power: when they were parted, it was evident to your senses that they had it, though they do not attract when they are together.
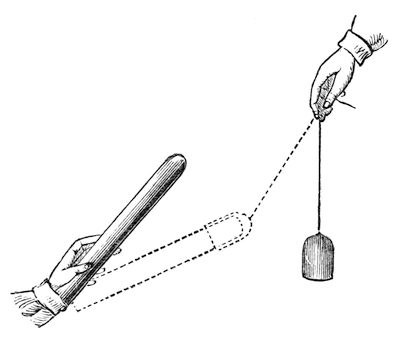
Fig. 34.
Fig. 35.
This, then, is sufficient in the outset to give you an idea of the nature of the force which we call ELECTRICITY. There is no end to the things from which you can evolve this power. When you go home, take a stick of sealing-wax – I have rather a large stick, but a smaller one will do – and make an indicator of this sort (fig. 35). Take a watch-glass (or your watch itself will do; you only want something which shall have a round face), and now, if you place a piece of flat glass upon that, you have a very easily moved centre. And if I take this lath and put it on the flat glass (you see I am searching for the centre of gravity of this lath – I want to balance it upon the watch-glass), it is very easily moved round; and if I take this piece of sealing-wax and rub it against my coat, and then try whether it is attractive [holding it near the lath], you see how strong the attraction is; I can even draw it about. Here, then, you have a very beautiful indicator, for I have, with a small piece of sealing-wax and my coat, pulled round a plank of that kind; so you need be in no want of indicators to discover the presence of this attraction. There is scarcely a substance which we may not use. Here are some indicators (fig. 36). I bend round a strip of paper into a hoop, and we have as good an indicator as can be required. See how it rolls along, travelling after the sealing-wax. If I make them smaller, of course we have them running faster, and sometimes they are actually attracted up into the air. Here also is a little collodion balloon. It is so electrical that it will scarcely leave my hand unless to go to the other. See, how curiously electrical it is: it is hardly possible for me to touch it without making it electrical; and here is a piece which clings to anything it is brought near, and which it is not easy to lay down. And here is another substance, gutta-percha, in thin strips: it is astonishing how, by rubbing this in your hands, you make it electrical. But our time forbids us to go further into this subject at present. You see clearly there are two kinds of electricities which may be obtained by rubbing shell-lac with flannel, or glass with silk.
Fig. 36.
Now, there are some curious bodies in nature (of which I have two specimens on the table) which are called magnets or loadstones– ores of iron, of which there is a great deal sent from Sweden. They have the attraction of gravitation, and attraction of cohesion, and certain chemical attraction; but they also have a great attractive power, for this little key is held up by this stone. Now, that is not chemical attraction, – it is not the attraction of chemical affinity, or of aggregation of particles, or of cohesion, or of electricity (for it will not attract this ball if I bring it near it); but it is a separate and dual attraction – and, what is more, one which is not readily removed from the substance, for it has existed in it for ages and ages in the bowels of the earth. Now, we can make artificial magnets (you will see me to-morrow make artificial magnets of extraordinary power). And let us take one of these artificial magnets, and examine it, and see where the power is in the mass, and whether it is a dual power. You see it attracts these keys, two or three in succession, and it will attract a very large piece of iron. That, then, is a very different thing indeed to what you saw in the case of the shell-lac; for that only attracted a light ball, but here I have several ounces of iron held up. And if we come to examine this attraction a little more closely, we shall find it presents some other remarkable differences: first of all, one end of this bar (fig. 37) attracts this key, but the middle does not attract. It is not, then, the whole of the substance which attracts. If I place this little key in the middle, it does not adhere; but if I place it there, a little nearer the end, it does, though feebly. Is it not, then, very curious to find that there is an attractive power at the extremities which is not in the middle – to have thus in one bar two places in which this force of attraction resides! If I take this bar and balance it carefully on a point, so that it will be free to move round, I can try what action this piece of iron has on it. Well, it attracts one end, and it also attracts the other end, just as you saw the shell-lac and the glass did, with the exception of its not attracting in the middle. But if now, instead of a piece of iron, I take a magnet, and examine it in a similar way, you see that one of its ends repels the suspended magnet – the force then is no longer attraction, but repulsion; but if I take the other end of the magnet and bring it near, it shews attraction again.

