 Полная версия
Полная версияOn the various forces of nature and their relations to each other
You see, therefore, how we are able, by using this electric spark, to get, first of all, the light into a very small space. That oil-lamp has a burner 3¾ inches in diameter. Compare the size of the flame with the space occupied by this electric light. Next, compare the intensity of this light with any other. If I take this candle, and place it by the side, I actually seem to put out the candle. We are thus able to get a light which, while it surpasses all others in brilliancy, is at the same time not too large; for I might put this light into an apparatus not larger than a hat, and yet I could count upon the rays being useful. Moreover, when such large burners are used in a lantern, we have to consider whether the bars of the window do not interfere to throw a shadow or otherwise; but with this light there will be no difficulty of that sort, as a single small speculum, no larger than a hat, will send it in any direction we please; and it is wonderful what advantages, by reason of its small bulk, we have in the consideration of the different kinds of apparatus required, reflecting or refracting, irrespective of other reasons for using the electric light. And it is these kind of things which make us decide most earnestly and carefully in favour of the electric light.
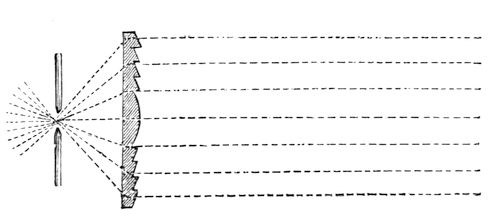
Fig. 58.
I am going to shew you the effect that will take place with that large lens, when we throw the oil-lamp out of action, and put the electric light into use. It is astonishing to find how little the eye can compare the relative intensities of two lights. Look at that screen, and try to recollect the amount of light thrown upon it from the 3¾ inch lamp of Fresnel; and, now, when we shift the lens sideways, look at the glorious light arising from that small carbon point (fig. 58) – see how beautifully it shines in the focus of that lens, and throws the rays forward. At present, the electric light is put at just the same distance as the oil light; and therefore, being in the focus of the lens, we have parallel rays which are thrown forward in a perfectly straight line – as you will see by comparing the size of the lens with that of the light thrown on the screen. You will now see how far we can affect this beam of light by increasing or diminishing the distance of the lamp. We are able, by a small adjustment, to get a beam of a large or small angle; and observe what power I have now over it, – for if I want to increase the degrees of divergence, I am limited by the power of light, in the case of the oil-lamp; but, with the electric light, I can make it spread over any width of the horizon by this simple adjustment. These, then, are some of the reasons which make it desirable to employ the electric light.
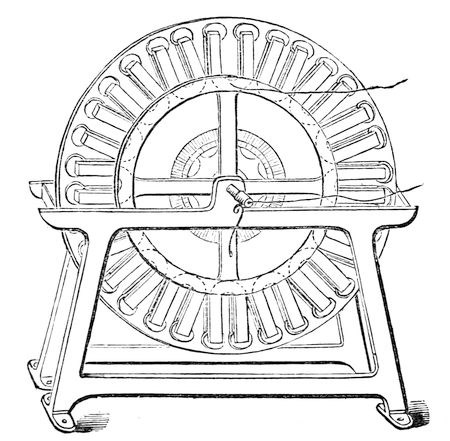
Fig. 59.
By means of a magnet, and of motion, we can get the same kind of electricity as I have here from the battery; and, under the authority of the Trinity House, Professor Holmes has been occupied in introducing the magneto-electric light in the light-house at the South Foreland; for the voltaic battery has been tried under every conceivable circumstance, and, I take the liberty of saying, it has hitherto proved a decided failure. Here, however, is an instrument wrought only by mechanical motion. The moment we give motion to this soft iron in front of the magnet, we get a spark. It is true, in this apparatus it is very small, but it is sufficient for you to judge of its character. It is the magneto-electric light; and an instrument has been constructed, as there shewn (fig. 59), which represents a number of magnets placed radially upon a wheel – three wheels of magnets and two sets of helices. When the machine, which is worked by a two-horse power engine, is properly set in motion, and the different currents are all brought together, and thrown by Professor Holmes up into the lantern, we have a light equal to the one we have been using this evening. For the last six months the South Foreland has been shining by means of this electric light – beyond all comparison, better than its former light. It has shone into France, and has been seen there and taken notice of by the authorities, who work with beautiful accord with us in all these matters. Never for once during six months has it failed in doing its duty – never once – more than was expected by the inventor. It has shone forth with its own peculiar character, and this even with the old apparatus; for, as yet, no attempt has been made to construct special reflectors or refractors for it, because it is not yet established. I will not tell you that the problem of employing the magneto-electric spark for light-house illumination is quite solved yet, although I desire it should be established most earnestly (for I regard this magnetic spark as one of my own offspring). The thing is not yet decidedly accomplished, and what the considerations of expense and other matters may be, I cannot tell. I am only here to tell you as a philosopher, how far the results have been carried; but I do hope that the authorities will find it a proper thing to carry out in full. If it cannot be introduced at all the light-houses – if it can only be used at one – why, really, it will be an honour to the nation which can originate such an improvement as this – one which must of necessity be followed by other nations.
You may ask, what is the use of this bright light? It would not be useful to us, were it not for the constant changes which are taking place in the atmosphere, which is never pure. Even when we can see the stars clearly on a bright night, it is not a pure atmosphere. The light of a light-house, more than any other, is liable to be dimmed by vapours and fogs; and where we most want this great power, is not in the finest condition of the atmosphere, but when the mariner is in danger – when the sleet and rain are falling, and the fogs arise, and the winds are blowing, and he is nearing coasts where the water is shallow, and abounds with rocks, – then is his time of danger, when he most wants this light. I am going to shew you how, by means of a little steam, I can completely obscure this glorious sun, this electric light which you see. The cloud now obscuring the light on the screen is only such a cloud as you see when sitting in a train on a fine summer’s day. You may observe that the vapour passing out of the funnel casts as deep a shadow on the ground as the black funnel; the very sun itself is extinguished by the steam from the funnel, so that it cannot give any light; and the sun itself, if set in the light-house, would not be able to penetrate such a vapour.
Now, the haze of this cloud of steam is just what we have to overcome, and the electric light is as soon, proportionally, extinguished by an obstruction of this kind as any other light. If we take two lights, one four times the intensity of the other, and we extinguish half of one by a vapour, we extinguish half of the other – and that is a fact which cannot be set aside by any arrangement. But, then, we fall back upon the amount of light which the electric spark does give us in aid of the power of penetrating the fog; for the light of the electric spark shines so far at times, that even before it has arisen above the horizon, twenty-five miles off, it can be seen. This intense light has, therefore, that power which we can take advantage of, – of bearing a great deal of obstruction, before it is entirely obscured by fogs or otherwise.
Taking care that we do not lead our authorities into error by the advice given, we hope that we shall soon be able to recommend the Trinity House, from what has passed, to establish either one or more good electric lights in this country.
1
Page 13. The opening lecture was twice postponed on account of Dr. Faraday’s illness.
2
Page 22. Platinum, with one exception, the heaviest body known, is 21½ times heavier than water.
3
Page 22. Aluminium is 2½ times heavier than water.
4
Pages 23 and 24. Power or Property in Water.– This power – the heat by which the water is kept in a fluid state – is said, under ordinary circumstances, to be latent or insensible. When, however, the water changes its form, and, by uniting with the lime or sulphate of copper, becomes solid, the heat which retained it in a liquid state is evolved.
5
Page 23. Anhydrous Sulphate of Copper: sulphate of copper deprived of its water of crystallisation. To obtain it, the blue sulphate is calcined in an earthen crucible.
6
Page 29. Add a little liquid to the marble, and decompose it.– Marble is composed of carbonic acid and lime, and, in chemical language, is called carbonate of lime. When sulphuric acid is added to it, the carbonic acid is set free, and the sulphuric acid unites with the lime to form sulphate of lime.
Carbonic acid, under ordinary circumstances, is a colourless invisible gas, about half as heavy again as air. Dr. Faraday first shewed that, under great pressure, it could be obtained in a liquid state. Thilorier, a French chemist, afterwards found that it could be solidified.
7
Page 55. Crystallisation of Alum.– The solution must be saturated – that is, it must contain as much alum as can possibly be dissolved. In making the solution, it is best to add powdered alum to hot water as long as it dissolves; and when no more is taken up, allow the solution to stand a few minutes, and then pour it off from the dirt and undissolved alum.
8
Page 57. Red Precipitate of Biniodide of Mercury.– A little care is necessary to obtain this precipitate. The solution of potassium should be added to the solution of perchloride of mercury (corrosive sublimate) very gradually. The red precipitate which first falls is redissolved when the liquid is stirred: when a little more of the iodide of potassium is added, a pale, red precipitate is formed, which, on the further addition of the iodide, changes into the brilliant scarlet biniodide of mercury. If too much iodide of potassium is added, the scarlet precipitate disappears, and a colourless solution is left.
9
Page 57. Paper Coated with Scarlet Biniodide of Mercury.– In order to fix the biniodide on paper, it must be mixed with a little weak gum water, and then spread over the paper, which must be dried without heat.
Biniodide of Mercury is said to be dimorphous; that is, is able to assume two different forms.
10
Page 59. “Prince Rupert’s Drops.”-These are made by pouring drops of melted green glass into cold water. They were not, as is commonly supposed, invented by Prince Rupert, but were first brought to England by him, in 1660. They excited a great deal of curiosity, and were considered “a kind of miracle in nature.”.
11
Page 60. Thick Glass Vessels.– They are called Proofs or Bologna phials.
12
Page 61. Mica.– A silicate of alumina and magnesia. It has a bright metallic lustre – hence its name, from mico, to shine.
13
Page 62. Common salt, or chloride of sodium, crystallises in the form of solid cubes, which, aggregated together, form a mass, which may be broken up into the separate cubes.
14
Page 62. Iceland or Calc Spar. – Native carbonate of lime in its primitive crystalline form.
15
Page 79. Solution of a Salt.– Acetate of soda. A solution saturated, or nearly so, at the boiling point, is necessary, and it must be allowed to cool, and remain at rest until the experiment is made.
16
Page 86. Binoxide of Nitrogen and Hypo-nitrous Acid.– Binoxide of nitrogen is formed when nitric acid and a little water are added to some copper turnings. It produces deep red fumes as soon as it comes in contact with the air, by combining with the oxygen of the latter to form hypo-nitrous acid. Binoxide of nitrogen is composed of two parts oxygen and one part of nitrogen; hypo-nitrous acid is composed of one part of nitrogen and three parts of oxygen.
17
ὕδωρ, “water,” and γενναω, “I generate.”.
18
Page 106. Chlorate of Potash and Sulphuret of Antimony.– Great care must be taken in mixing these substances, as the mixture is dangerously explosive. They must be powdered separately, and mixed together with a feather on a sheet of paper, or by passing them several times through a small sieve.
19
Page 107. The mixture of chlorate of potash and sugar does not require the same precautions. They may be rubbed together in a pestle and mortar without fear. One part of chlorate of potash and three parts of sugar will answer. The mixture need only be touched with a glass rod dipped in oil of vitriol.
20
Page 107. Two Salts Dissolved in Water.– Sulphate of soda and chloride of calcium. The solutions must be saturated for the experiment to succeed well.
21
Page 111. Lead Pyrophorous.– This is a tartrate of lead which has been heated in a glass tube to dull redness as long as vapours are emitted. As soon as they cease to be evolved, the end of the tube is sealed, and it is allowed to cool.
22
Page 115. Gun-Cotton is made by immersing cotton-wool in a mixture of sulphuric acid and the strongest nitric acid, or of sulphuric acid and nitrate of potash.
23
Page 115. Paper Prepared like Gun-Cotton.– It should be bibulous paper, and must be soaked for ten minutes in a mixture of ten parts by measure of oil of vitriol with five parts of strong fuming nitric acid. The paper must afterwards be thoroughly washed with warm distilled water, and then carefully dried at a gentle heat. The paper is then saturated with chlorate of strontia, or chlorate of baryta, or nitrate of copper, by immersion in a warm solution of these salts. (See Chemical News, Vol. I., page 36.)
24
Page 162. Sulpho-indigotic Acid.– A mixture of one part of indigo and fifteen parts of concentrated oil of vitriol. It is bleached on the side at which hydrogen gas is evolved, in consequence of the liberated hydrogen withdrawing oxygen from the indigo, thereby forming a colourless deoxidised indigo. In making the experiment, only enough of the sulpho-indigotic acid must be added to give the water a decided blue colour.
25
Page 164. Lead Tree.– To make a lead tree, pass a bundle of brass wires through the cork of a bottle, and fasten a plate of zinc round them just as they issue from the cork, so that the zinc may be in contact with every one of the wires. Make the wires to diverge so as to form a sort of cone, and having filled the bottle quite full of a solution of sugar of lead, insert the wires and cork, and seal it down, so as to perfectly exclude the air. In a short time the metallic lead will begin to crystallise around the divergent wires, and form a beautiful object.

