 Полная версия
Полная версияOn the various forces of nature and their relations to each other
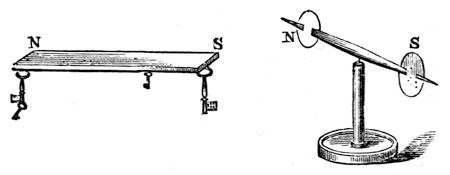
Fig. 37. and Fig. 38.
You will see this better, perhaps, by another kind of experiment. Here (fig. 38) is a little magnet, and I have coloured the ends differently, so that you may distinguish one from the other. Now this end (S) of the magnet (fig. 37) attracts the uncoloured end of the little magnet. You see it pulls it towards it with great power; and as I carry it round, the uncoloured end still follows. But now, if I gradually bring the middle of the bar magnet opposite the uncoloured end of the needle, it has no effect upon it, either of attraction or repulsion, until, as I come to the opposite extremity (N), you see that it is the coloured end of the needle which is pulled towards it. We are now therefore dealing with two kinds of power, attracting different ends of the magnet – a double power, already existing in these bodies, which takes up the form of attraction and repulsion. And now, when I put up this label with the word MAGNETISM, you will understand that it is to express this double power.
Now, with this loadstone you may make magnets artificially. Here is an artificial magnet (fig. 39) in which both ends have been brought together in order to increase the attraction. This mass will lift that lump of iron; and, what is more, by placing this keeper, as it is called, on the top of the magnet, and taking hold of the handle, it will adhere sufficiently strongly to allow itself to be lifted up – so wonderful is its power of attraction. If you take a needle, and just draw one of its ends along one extremity of the magnet, and then draw the other end along the other extremity, and then gently place it on the surface of some water (the needle will generally float on the surface, owing to the slight greasiness communicated to it by the fingers), you will be able to get all the phenomena of attraction and repulsion, by bringing another magnetised needle near to it.
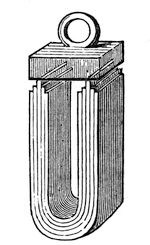
Fig. 39.
I want you now to observe, that although I have shewn you in these magnets that this double power becomes evident principally at the extremities, yet the whole of the magnet is concerned in giving the power. That will at first seem rather strange; and I must therefore shew you an experiment to prove that this is not an accidental matter, but that the whole of the mass is really concerned in this force, just as in falling the whole of the mass is acted upon by the force of gravitation. I have here (fig. 40) a steel bar, and I am going to make it a magnet, by rubbing it on the large magnet (fig. 39). I have now made the two ends magnetic in opposite ways. I do not at present know one from the other, but we can soon find out. You see when I bring it near our magnetic needle (fig. 38) one end repels and the other attracts; and the middle will neither attract nor repel – it cannot, because it is half-way between the two ends. But now, if I break out that piece (n s), and then examine it – see how strongly one end (n) pulls at this end (S, fig. 38), and how it repels the other end (N). And so it can be shewn that every part of the magnet contains this power of attraction and repulsion, but that the power is only rendered evident at the end of the mass. You will understand all this in a little while; but what you have now to consider is, that every part of this steel is in itself a magnet. Here is a little fragment which I have broken out of the very centre of the bar, and you will still see that one end is attractive and the other is repulsive. Now, is not this power a most wonderful thing? and very strange the means of taking it from one substance and bringing it to other matters? I cannot make a piece of iron or anything else heavier or lighter than it is. Its cohesive power it must and does have; but, as you have seen by these experiments, we can add or subtract this power of magnetism, and almost do as we like with it.
Fig. 40.
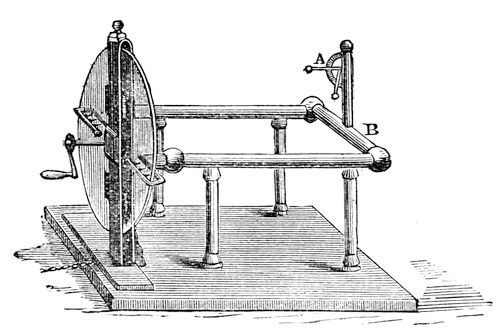
Fig. 41.
And now we will return for a short time to the subject treated of at the commencement of this lecture. You see here (fig. 41) a large machine, arranged for the purpose of rubbing glass with silk, and for obtaining the power called electricity; and the moment the handle of the machine is turned, a certain amount of electricity is evolved, as you will see by the rise of the little straw indicator (at A). Now, I know from the appearance of repulsion of the pith ball at the end of the straw, that electricity is present in those brass conductors (B B), and I want you to see the manner in which that electricity can pass away. [Touching the conductor (B) with his finger, the Lecturer drew a spark from it, and the straw electrometer immediately fell.] There, it has all gone; and that I have really taken it away, you shall see by an experiment of this sort. If I hold this cylinder of brass by the glass handle, and touch the conductor with it, I take away a little of the electricity. You see the spark in which it passes, and observe that the pith-ball indicator has fallen a little, which seems to imply that so much electricity is lost; but it is not lost: it is here in this brass; and I can take it away and carry it about, not because it has any substance of its own, but by some strange property which we have not before met with as belonging to any other force. Let us see whether we have it here or not. [The Lecturer brought the charged cylinder to a jet from which gas was issuing; the spark was seen to pass from the cylinder to the jet, but the gas did not light.] Ah! the gas did not light, but you saw the spark; there is, perhaps, some draught in the room which blew the gas on one side, or else it would light. We will try this experiment afterwards. You see from the spark that I can transfer the power from the machine to this cylinder, and then carry it away and give it to some other body. You know very well, as a matter of experiment, that we can transfer the power of heat from one thing to another; for if I put my hand near the fire it becomes hot. I can shew you this by placing before us this ball, which has just been brought red-hot from the fire. If I press this wire to it, some of the heat will be transferred from the ball; and I have only now to touch this piece of gun-cotton with the hot wire, and you see how I can transfer the heat from the ball to the wire, and from the wire to the cotton. So you see that some powers are transferable, and others are not. Observe how long the heat stops in this ball. I might touch it with the wire, or with my finger, and if I did so quickly, I should merely burn the surface of the skin; whereas, if I touch that cylinder, however rapidly, with my finger, the electricity is gone at once – dispersed on the instant, in a manner wonderful to think of.
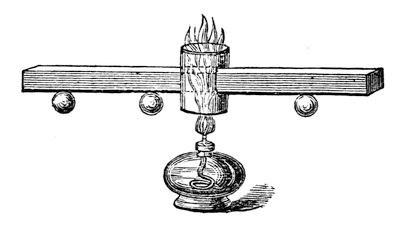
Fig. 42.
I must now take up a little of your time in shewing you the manner in which these powers are transferred from one thing to another; for the manner in which force may be conducted or transmitted is extraordinary, and most essential for us to understand. Let us see in what manner these powers travel from place to place. Both heat and electricity can be conducted; and here is an arrangement I have made to shew how the former can travel. It consists of a bar of copper (fig. 42); and if I take a spirit-lamp (this is one way of obtaining the power of heat), and place it under that little chimney, the flame will strike against the bar of copper and keep it hot. Now, you are aware that power is being transferred from the flame of that lamp to the copper, and you will see by-and-by that it is being conducted along the copper from particle to particle; for, inasmuch as I have fastened these wooden balls by a little wax at particular distances from the point where the copper is first heated, first one ball will fall, and then the more distant ones, as the heat travels along – and thus you will learn that the heat travels gradually through the copper. You will see that this is a very slow conduction of power, as compared with electricity. If I take cylinders of wood and metal, joined together at the ends, and wrap a piece of paper round, and then apply the heat of this lamp to the place where the metal and wood join, you will see how the heat will accumulate where the wood is, and burn the paper with which I have covered it; but where the metal is beneath, the heat is conducted away too fast for the paper to be burned. And so, if I take a piece of wood and a piece of metal joined together, and put it so that the flame should play equally both upon one and the other, we shall soon find that the metal will become hot before the wood; for if I put a piece of phosphorus on the wood, and another piece on the copper, you will find that the phosphorus on the copper will take fire before that on the wood is melted – and this shews you how badly the wood conducts heat. But with regard to the travelling of electricity from place to place, its rapidity is astonishing. I will, first of all, take these pieces of glass and metal, and you will soon understand how it is that the glass does not lose the power which it acquired when it is rubbed by the silk. By one or two experiments I will shew you. If I take this piece of brass and bring it near the machine, you see how the electricity leaves the latter, and passes to the brass cylinder. And, again, if I take a rod of metal and touch the machine with it, I lower the indicator; but when I touch it with a rod of glass, no power is drawn away, – shewing you that the electricity is conducted by the glass and the metal in a manner entirely different: and to make you see that more clearly, we will take one of our Leyden jars. Now, I must not embarrass your minds with this subject too much; but if I take a piece of metal, and bring it against the knob at the top and the metallic coating at the bottom, you will see the electricity passing through the air as a brilliant spark. It takes no sensible time to pass through this; and if I were to take a long metallic wire, no matter what the length – at least as far as we are concerned – and if I make one end of it touch the outside, and the other touch the knob at the top, see how the electricity passes! – it has flashed instantaneously through the whole length of this wire. Is not this different from the transmission of heat through this copper bar (fig. 42), which has taken a quarter of an hour or more to reach the first ball?

Fig. 43.
Here is another experiment, for the purpose of shewing the conductibility of this power through some bodies, and not through others. Why do I have this arrangement made of brass? [pointing to the brass work of the electrical machine, fig. 41]. Because it conducts electricity. And why do I have these columns made of glass? Because they obstruct the passage of electricity. And why do I put that paper tassel (fig. 43) at the top of the pole, upon a glass rod, and connect it with this machine by means of a wire? You see at once that as soon as the handle of the machine is turned, the electricity which is evolved travels along this wire and up the wooden rod, and goes to the tassel at the top, and you see the power of repulsion with which it has endowed these strips of paper, each spreading outwards to the ceiling and sides of the room. The outside of that wire is covered with gutta-percha. It would not serve to keep the force from you when touching it with your hands, because it would burst through; but it answers our purpose for the present. And so you perceive how easily I can manage to send this power of electricity from place to place, by choosing the materials which can conduct the power. Suppose I want to fire a portion of gunpowder, I can readily do it by this transferable power of electricity. I will take a Leyden jar, or any other arrangement which gives us this power, and arrange wires so that they may carry the power to the place I wish; and then placing a little gunpowder on the extremities of the wires, the moment I make the connection by this discharging rod, I shall fire the gunpowder. [The connection was made, and the gunpowder ignited.] And if I were to shew you a stool like this, and were to explain to you its construction, you could easily understand that we use glass legs, because these are capable of preventing the electricity from going away to the earth. If, therefore, I were to stand on this stool, and receive the electricity through this conductor, I could give it to anything that I touched. [The Lecturer stood upon the insulating stool, and placed himself in connection with the conductor of the machine.] Now, I am electrified – I can feel my hair rising up as the paper tassel did just now. Let us see whether I can succeed in lighting gas by touching the jet with my finger. [The Lecturer brought his finger near a jet from which gas was issuing, when, after one or two attempts, the spark which came from his finger to the jet set fire to the gas.] You now see how it is that this power of electricity can be transferred from the matter in which it is generated, and conducted along wires and other bodies, and thus be made to serve new purposes utterly unattainable by the powers we have spoken of on previous days; and you will not now be at a loss to bring this power of electricity into comparison with those which we have previously examined; and to-morrow we shall be able to go further into the consideration of these transferable powers.
LECTURE VI.
THE CORRELATION OF THE PHYSICAL FORCES
We have frequently seen, during the course of these lectures, that one of those powers or forces of matter, of which I have written the names on that board, has produced results which are due to the action of some other force. Thus, you have seen the force of electricity acting in other ways than in attracting: you have also seen it combine matters together, or disunite them, by means of its action on the chemical force; and in this case, therefore, you have an instance in which these two powers are related. But we have other and deeper relations than these; we have not merely to see how it is that one power affects another – how the force of heat affects chemical affinity, and so forth – but we must try and comprehend what relation they bear to each other, and how these powers may be changed one into the other; and it will to-day require all my care, and your care too, to make this clear to your minds. I shall be obliged to confine myself to one or two instances, because, to take in the whole extent of this mutual relation and conversion of forces, would surpass the human intellect.
In the first place, then, here is a piece of fine zinc-foil; and if I cut it into narrow strips and apply to it the power of heat, admitting the contact of air at the same time, you will find that it burns; and then, seeing that it burns, you will be prepared to say that there is chemical action taking place. You see all I have to do is to hold the piece of zinc at the side of the flame, so as to let it get heated, and yet to allow the air which is flowing into the flame from all sides to have access to it; – there is the piece of zinc burning just like a piece of wood, only brighter. A part of the zinc is going up into the air, in the form of that white smoke, and part is falling down on to the table. This, then, is the action of chemical affinity exerted between the zinc and the oxygen of the air. I will shew you what a curious kind of affinity this is by an experiment, which is rather striking when seen for the first time. I have here some iron filings and gunpowder, and will mix them carefully together, with as little rough handling as possible. Now, we will compare the combustibility, so to speak, of the two. I will pour some spirit of wine into a basin, and set it on fire: and, having our flame, I will drop this mixture of iron filings and gunpowder through it, so that both sets of particles will have an equal chance of burning. And now, tell me which of them it is that burns? You see a plentiful combustion of the iron-filings. But I want you to observe that, though they have equal chances of burning, we shall find that by far the greater part of the gunpowder remains untouched. I have only to drain off this spirit of wine, and let the powder which has gone through the flame dry, which it will do in a few minutes, and I will then test it with a lighted match. So ready is the iron to burn, that it takes, under certain circumstances, even less time to catch fire than gunpowder. [As soon as the gunpowder was dry, Mr. Anderson handed it to the Lecturer, who applied a lighted match to it, when a sudden flash shewed how large a proportion of gunpowder had escaped combustion when falling through the flame of alcohol.]
These are all cases of chemical affinity; and I shew them to make you understand that we are about to enter upon the consideration of a strange kind of chemical affinity, and then to see how far we are enabled to convert this force of affinity into electricity or magnetism, or any other of the forces which we have discussed. Here is some zinc (I keep to the metal zinc, as it is very useful for our purpose), and I can produce hydrogen gas by putting the zinc and sulphuric acid together, as they are in that retort. There you see the mixture which gives us hydrogen – the zinc is pulling the water to pieces and setting free hydrogen gas. Now, we have learned by experience that, if a little mercury is spread over that zinc, it does not take away its power of decomposing the water, but modifies it most curiously. See how that mixture is now boiling; but when I add a little mercury to it, the gas ceases to come off. We have now scarcely a bubble of hydrogen set free, so that the action is suspended for the time. We have not destroyed the power of chemical affinity, but modified it in a wonderful and beautiful manner. Here are some pieces of zinc covered with mercury, exactly in the same way as the zinc in that retort is covered; and if I put this plate into sulphuric acid, I get no gas – but this most extraordinary thing occurs, that if I introduce along with the zinc another metal which is not so combustible, then I reproduce all the action. I am now going to put to the amalgamated zinc in this retort some portions of copper wire (copper not being so combustible a metal as the zinc), and observe how I get hydrogen again. As in the first instance, there the bubbles are coming over through the pneumatic trough, and ascending faster and faster in the jar. The zinc now is acting by reason of its contact with the copper.
Every step we are now taking brings us to a knowledge of new phenomena. That hydrogen which you now see coming off so abundantly does not come from the zinc, as it did before, but from the copper. Here is a jar containing a solution of copper. If I put a piece of this amalgamated zinc into it, and leave it there, it has scarcely any action; and here is a plate of platinum, which I will immerse in the same solution, and might leave it there for hours, days, months, or even years, and no action would take place. But by putting them both together, and allowing them to touch (fig. 44), you see what a coating of copper there is immediately thrown down on the platinum. Why is this? The platinum has no power of itself to reduce that metal from that fluid, but it has in some mysterious way received this power by its contact with the metal zinc. Here, then, you see a strange transfer of chemical force from one metal to another – the chemical force from the zinc is transferred, and made over to the platinum by the mere association of the two metals. I might take, instead of the platinum, a piece of copper or of silver, and it would have no action of its own on this solution; but the moment the zinc was introduced and touched the other metal, then the action would take place, and it would become covered with copper. Now, is not this most wonderful and beautiful to see? We still have the identical chemical force of the particles of zinc acting, and yet in some strange manner we have power to make that chemical force, or something it produces, travel from one place to another – for we do make the chemical force travel from the zinc to the platinum by this very curious experiment of using the two metals in the same fluid in contact with each other.
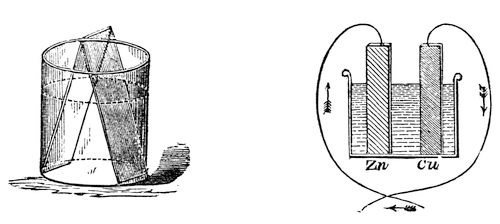
Fig. 44. and Fig. 45.
Let us now examine these phenomena a little more closely. Here is a drawing (fig. 45) in which I have represented a vessel containing the acid liquid, and the slips of zinc and platinum or copper, and I have shewn them touching each other outside by means of a wire coming from each of them (for it matters not whether they touch in the fluid or outside – by pieces of metal attached – they still by that communication between them have this power transferred from one to another). Now, if instead of only using one vessel, as I have shewn there, I take another, and another, and put in zinc and platinum, zinc and platinum, zinc and platinum, and connect the platinum of one vessel with the zinc of another, the platinum of this vessel with the zinc of that, and so on, we should only be using a series of these vessels instead of one. This we have done in that arrangement which you see behind me. I am using what we call a Grove’s voltaic battery, in which one metal is zinc, and the other platinum, and I have as many as forty pairs of these plates all exercising their force at once in sending the whole amount of chemical power there evolved through these wires under the floor, and up to these two rods coming through the table. We need do no more than just bring these two ends in contact, when the spark shews us what power is present; and what a strange thing it is to see that this force is brought away from the battery behind me, and carried along through these wires. I have here an apparatus (fig. 46) which Sir Humphry Davy constructed many years ago, in order to see whether this power from the voltaic battery caused bodies to attract each other in the same manner as the ordinary electricity did. He made it in order to experiment with his large voltaic battery, which was the most powerful then in existence. You see there are in this glass jar two leaves of gold, which I can cause to move to and fro by this rack-work. I will connect each of these gold leaves with separate ends of this battery; and, if I have a sufficient number of plates in the battery, I shall be able to shew you that there will be some attraction between those leaves, even before they come in contact. If I bring them sufficiently near when they are in communication with the ends of the battery, they will be drawn gently together; and you will know when this takes place, because the power will cause the gold leaves to burn away, which they could only do when they touched each other. Now, I am going to cause these two leaves of gold to approach gradually, and I have no doubt that some of you will see that they approach before they burn; and those who are too far off to see them approach will see by their burning that they have come together. Now they are attracting each other, long before the connection is complete; and there they go! burnt up in that brilliant flash – so strong is the force. You thus see, from the attractive force at the two ends of this battery, that these are really and truly electrical phenomena.
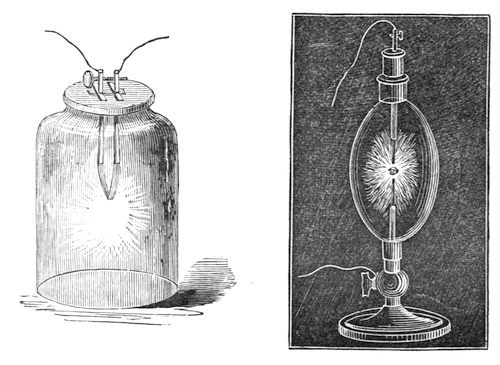
Fig. 46. and Fig. 47.
Now, let us consider what is this spark. I take these two ends and bring them together, and there I get this glorious spark, like the sunlight in the heavens above us. What is this? It is the same thing which you saw when I discharged the large electrical machine, when you saw one single bright flash; it is the same thing, only continued, because here we have a more effective arrangement. Instead of having a machine which we are obliged to turn for a long time together, we have here a chemical power which sends forth the spark; and it is wonderful and beautiful to see how this spark is carried about through these wires. I want you to perceive, if possible, that this very spark and the heat it produces (for there is heat) is neither more nor less than the chemical force of the zinc – its very force carried along wires and conveyed to this place. I am about to take a portion of the zinc and burn it in oxygen gas, for the sake of shewing you the kind of light produced by the actual combustion in oxygen gas of some of this metal. [A tassel of zinc-foil was ignited at a spirit-lamp, and introduced into a jar of oxygen, when it burnt with a brilliant light.] That shews you what the affinity is when we come to consider it in its energy and power. And the zinc is being burned in the battery behind me at a much more rapid rate than you see in that jar, because the zinc is there dissolving and burning, and produces here this great electric light. That very same power, which in that jar you saw evolved from the actual combustion of the zinc in oxygen, is carried along these wires and made evident here; and you may, if you please, consider that the zinc is burning in those cells, and that this is the light of that burning [bringing the two poles in contact, and shewing the electric light]; and we might so arrange our apparatus as to shew that the amounts of power evolved in either case are identical. Having thus obtained power over the chemical force, how wonderfully we are able to convey it from place to place! When we use gunpowder for explosive purposes, we can send into the mine chemical affinity by means of this electricity; not having provided fire beforehand, we can send it in at the moment we require it. Now, here (fig. 47) is a vessel containing two charcoal points, and I bring it forward as an illustration of the wonderful power of conveying this force from place to place. I have merely to connect these by means of wires to the opposite ends of the battery, and bring the points in contact. See what an exhibition of force we have! We have exhausted the air so that the charcoal cannot burn; and, therefore, the light you see is really the burning of the zinc in the cells behind me – there is no disappearance of the carbon, although we have that glorious electric light; and the moment I cut off the connection, it stops. Here is a better instance to enable some of you to see the certainty with which we can convey this force, where, under ordinary circumstances, chemical affinity would not act. We may absolutely take these two charcoal poles down under water, and get our electric light there; – there they are in the water, and you observe, when I bring them into connection, we have the same light as we had in that glass vessel.

