 Полная версия
Полная версияOn the various forces of nature and their relations to each other
You have now seen that the solid water can become fluid by the addition of heat, owing to this lessening the attractive force between its particles, and yet you see that there is a good deal of attractive force remaining behind. I want now to take you another step beyond. We saw that if we continued applying heat to the water (as indeed happened with our piece of ice here), that we did at last break up that attraction which holds the liquid together; and I am about to take some ether (any other liquid would do, but ether makes a better experiment for my purpose), in order to illustrate what will happen when this cohesion is broken up. Now, this liquid ether, if exposed to a very low temperature, will become a solid; but if we apply heat to it, it becomes vapour, and I want to shew you the enormous bulk of the substance in this new form – when we make ice into water, we lessen its bulk, but when we convert water into steam, we increase it to an enormous extent. You see it is very clear that as I apply heat to the liquid I diminish its attraction of cohesion – it is now boiling, and I will set fire to the vapour, so that you may be enabled to judge of the space occupied by the ether in this form by the size of its flame, and you now see what an enormously bulky flame I get from that small volume of ether below. The heat from the spirit-lamp is now being consumed, not in making the ether any warmer, but in converting it into vapour; and if I desired to catch this vapour and condense it (as I could without much difficulty), I should have to do the same as if I wished to convert steam into water and water into ice: in either case it would be necessary to increase the attraction of the particles, by cold or otherwise. So largely is the bulk occupied by the particles increased by giving them this diminished attraction, that if I were to take a portion of water a cubic inch in bulk (A, fig. 23) I should produce a volume of steam of that size, B [1700 cubic inches; nearly a cubic foot], so greatly is the attraction of cohesion diminished by heat; and yet it still remains water. You can easily imagine the consequences which are due to this change in volume by heat – the mighty powers of steam and the tremendous explosions which are sometimes produced by this force of water. I want you now to see another experiment, which will perhaps give you a better illustration of the bulk occupied by a body when in the state of vapour. Here is a substance which we call iodine, and I am about to submit this solid body to the same kind of condition as regards heat that I did the water and the ether [putting a few grains of iodine into a hot glass globe, which immediately became filled with the violet vapour], and you see the same kind of change produced. Moreover, it gives us the opportunity of observing how beautiful is the violet-coloured vapour from this black substance, or rather the mixture of the vapour with air (for I would not wish you to understand that this globe is entirely filled with the vapour of iodine).
If I had taken mercury and converted it into vapour (as I could easily do), I should have a perfectly colourless vapour; for you must understand this about vapours, that bodies in what we call the vaporous, or the gaseous state, are always perfectly transparent, never cloudy or smoky: they are, however, often coloured, and we can frequently have coloured vapours or gases produced by colourless particles themselves mixing together, as in this case [the Lecturer here inverted a glass cylinder full of binoxide of nitrogen16 over a cylinder of oxygen, when the dark-red vapour of hypo-nitrous acid was produced]. Here also you see a very excellent illustration of the effect of a power of nature which we have not as yet come to, but which stands next on our list – Chemical Affinity. And thus you see we can have a violet vapour or an orange vapour, and different other kinds of vapour; but they are always perfectly transparent, or else they would cease to be vapours.
I am now going to lead you a step beyond this consideration of the attraction of the particles for each other. You see we have come to understand that, if we take water as an illustration, whether it be ice, or water, or steam, it is always to be considered by us as water. Well, now prepare your minds to go a little deeper into the subject. We have means of searching into the constitution of water beyond any that are afforded us by the action of heat, and among these one of the most important is that force which we call voltaic electricity, which we used at our last meeting for the purpose of obtaining light, and which we carried about the room by means of these wires. This force is produced by the battery behind me, to which, however, I will not now refer more particularly: before we have done we shall know more about this battery, but it must grow up in our knowledge as we proceed. Now, here (fig. 24) is a portion of water in this little vessel C, and besides the water there are two plates of the metal platinum, which are connected with the wires (A and B) coming outside, and I want to examine that water, and the state and the condition in which its particles are arranged. If I were to apply heat to it, you know what we should get; it would assume the state of vapour, but it would nevertheless remain water, and would return to the liquid state as soon as the heat was removed. Now, by means of these wires (which are connected with the battery behind me, and come under the floor and up through the table), we shall have a certain amount of this new power at our disposal. Here you see it is [causing the ends of the wires to touch] – that is the electric light we used yesterday, and by means of these wires we can cause water to submit itself to this power; for the moment I put them into metallic connection (at A and B), you see the water boiling in that little vessel (C), and you hear the bubbling of the gas that is going through the tube (D). See how I am converting the water into vapour; and if I take a little vessel (E), and fill it with water, and put it in the trough over the end of the tube (D), there goes the vapour ascending into the vessel. And yet that is not steam; for you know that if steam is brought near cold water, it would at once condense, and return back again to water. This then cannot be steam, for it is bubbling through the cold water in this trough; but it is a vaporous substance, and we must therefore examine it carefully, to see in what way the water has been changed. And now, in order to give you a proof that it is not steam, I am going to shew you that it is combustible; for if I take this small vessel to a light, the vapour inside explodes in a manner that steam could never do.
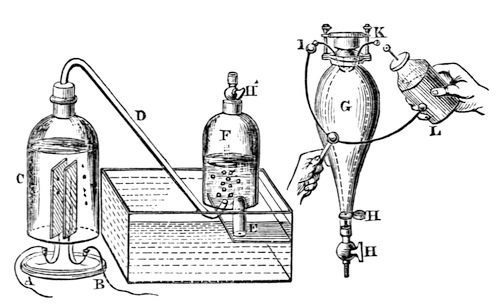
Fig. 24.
I will now fill this large bell-jar (F) with water; and I propose letting the gas ascend into it, and I will then shew you that we can reproduce the water back again from the vapour or air that is there. Here is a strong glass vessel (G), and into it we will let the gas (from F) pass. We will there fire it by the electric spark, and then after the explosion you will find that we have got the water back again: it will not be much, however, for you will recollect that I shewed you how small a portion of water produced a very large volume of vapour. Mr. Anderson will now pump all the air out of this vessel (G); and when I have screwed it on to the top of our jar of gas (F), you will see upon opening the stop-cocks (H´ H H) the water will jump up, shewing that some of the gas has passed into the glass vessel. I will now shut these stop-cocks, and we shall be able to send the electric spark through the gas by means of the wires (I, K) in the upper part of the vessel, and you will see it burn with a most intense flash. [Mr. Anderson here brought a Leyden jar, which he discharged through the confined gas by means of the wires I, K.] You saw the flash; and now that you may see that there is no longer any gas remaining, if I place it over the jar and open the stop-cocks again, up will go the gas, and we can have a second combustion; and so I might go on again and again, and I should continue to accumulate more and more of the water to which the gas has returned. Now, is not this curious? – in this vessel (C) we can go on making from water a large bulk of permanent gas, as we call it, and then we can reconvert it into water in this way. [Mr. Anderson brought in another Leyden jar, which, however, from some cause would not ignite the gas. It was therefore recharged, when the explosion took place in the desired manner.] How beautifully we get our results when we are right in our proceedings! – it is not that Nature is wrong when we make a mistake. Now, I will lay this vessel (G) down by my right hand, and you can examine it by and by: there is not very much water flowing down, but there is quite sufficient for you to see.
Another wonderful thing about this mode of changing the condition of the water is this – that we are able to get the separate parts of which it is composed, at a distance the one from the other, and to examine them, and see what they are like, and how many of them there are; and for this purpose I have here some more water in a slightly different apparatus to the former one (fig. 25), and if I place this in connection with the wires of the battery (at A B), I shall get a similar decomposition of the water at the two platinum plates. Now, I will put this little tube (O) over there, and that will collect the gas together that comes from this side (A), and this tube (H) will collect the gas that comes from the other side (B); and I think we shall soon be able to see a difference. In this apparatus the wires are a good way apart from each other, and it now seems that each of them is capable of drawing off particles from the water and sending them off, and you see that one set of particles (H) is coming off twice as fast as those collected in the other tube (O). Something is coming out of the water there (at H) which burns [setting fire to the gas]; but what comes out of the water here (at O), although it will not burn, will support combustion very vigorously. [The Lecturer here placed a match with a glowing tip in the gas, when it immediately rekindled.]
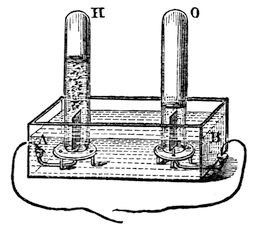
Fig. 25.
Here, then, we have two things, neither of them being water alone, but which we get out of the water. Water is therefore composed of two substances different to itself, which appear at separate places when it is made to submit to the force which I have in these wires; and if I take an inverted tube of water and collect this gas (H), you will see that it is by no means the same as the one we collected in the former apparatus (fig. 24). That exploded with a loud noise when it was lighted, but this will burn quite noiselessly – it is called hydrogen; and the other we call oxygen– that gas which so beautifully brightens up all combustion, but does not burn of itself. So now we see that water consists of two kinds of particles attracting each other in a very different manner to the attraction of gravitation or cohesion; and this new attraction we call chemical affinity, or the force of chemical action between different bodies. We are now no longer concerned with the attraction of iron for iron, water for water, wood for wood, or like bodies for each other, as we were when dealing with the force of cohesion: we are dealing with another kind of attraction, – the attraction between particles of a different nature one to the other. Chemical affinity depends entirely upon the energy with which particles of different kinds attract each other. Oxygen and hydrogen are particles of different kinds, and it is their attraction to each other which makes them chemically combine and produce water.
I must now shew you a little more at large what chemical affinity is. I can prepare these gases from other substances, as well as from water; and we will now prepare some oxygen. Here is another substance which contains oxygen – chlorate of potash. I will put some of it into this glass retort, and Mr. Anderson will apply heat to it. We have here different jars filled with water; and when, by the application of heat, the chlorate of potash is decomposed, we will displace the water, and fill the jars with gas.
Now, when water is opened out in this way by means of the battery – which adds nothing to it materially, which takes nothing from it materially (I mean no matter; I am not speaking of force), which adds no matter to the water – it is changed in this way: the gas which you saw burning a little while ago, called hydrogen, is evolved in large quantity, and the other gas, oxygen, is evolved in only half the quantity; so that these two areas represent water, and these are always the proportions between the two gases.
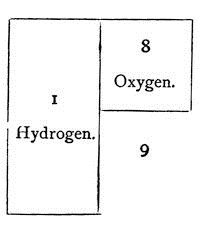
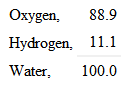
But oxygen is sixteen times the weight of the other – eight times as heavy as the particles of hydrogen in the water; and you therefore know that water is composed of nine parts by weight – one of hydrogen and eight of oxygen; thus: —
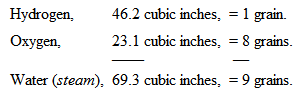
Now, Mr. Anderson has prepared some oxygen, and we will proceed to examine what is the character of this gas. First of all, you remember, I told you that it does not burn, but that it affects the burning of other bodies. I will just set fire to the point of this little bit of wood, and then plunge it into the jar of oxygen, and you will see what this gas does in increasing the brilliancy of the combustion. It does not burn – it does not take fire as the hydrogen would – but how vividly the combustion of the match goes on. Again, if I were to take this wax taper and light it, and turn it upside down in the air, it would in all probability put itself out, owing to the wax running down into the wick. [The Lecturer here turned the lighted taper upside down, when in a few seconds it went out.] Now, that will not happen in oxygen gas; you will see how differently it acts (fig. 26). [The taper was again lighted, turned upside down, and then introduced into a jar of oxygen.] Look at that! see how the very wax itself burns, and falls down in a dazzling stream of fire, so powerfully does the oxygen support combustion. Again, here is another experiment which will serve to illustrate the force, if I may so call it, of oxygen. I have here a circular flame of spirit of wine, and with it I am about to shew you the way in which iron burns, because it will serve very well as a comparison between the effect produced by air and oxygen. If I take this ring flame, I can shake by means of a sieve the fine particles of iron filings through it, and you will see the way in which they burn. [The Lecturer here shook through the flame some iron filings, which took fire and fell through with beautiful scintillations.] But if I now hold the flame over a jar of oxygen [the experiment was repeated over a jar of oxygen, when the combustion of the filings, as they fell into the oxygen, became almost insupportably brilliant], you see how wonderfully different the effect is in the jar; because there we have oxygen instead of common air.
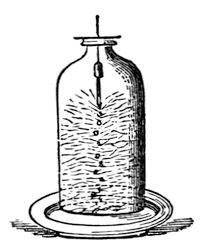
Fig. 26.
LECTURE IV.
CHEMICAL AFFINITY – HEAT
We shall have to pay a little more attention to the forces existing in water before we can have a clear idea on the subject. Besides the attraction which there is between its particles to make it hold together as a liquid or a solid, there is also another force, different from the former – one which, yesterday, by means of the voltaic battery, we overcame, drawing from the water two different substances – which, when heated by means of the electric spark, attracted each other, and rushed into combination to reproduce water. Now, I propose to-day to continue this subject, and trace the various phenomena of chemical affinity; and for this purpose, as we yesterday considered the character of oxygen, of which I have here two jars (oxygen being those particles derived from the water which enable other bodies to burn), we will now consider the other constituent of water; and, without embarrassing you too much with the way in which these things are made, I will proceed now to shew you our common way of making hydrogen. (I called it hydrogen yesterday – it is so called because it helps to generate water.)17 I put into this retort some zinc, water, and oil of vitriol, and immediately an action takes place, which produces an abundant evolution of gas, now coming over into this jar, and bubbling up in appearance exactly like the oxygen we obtained yesterday.
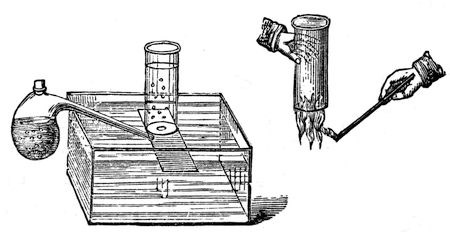
Fig. 27.
The processes, you see, are very different, though the result is the same, in so far as it gives us certain gaseous particles. Here, then, is the hydrogen. I shewed you yesterday certain qualities of this gas; now let me exhibit you some other properties. Unlike oxygen, which is a supporter of combustion, and will not burn, hydrogen itself is combustible. There is a jar full of it; and if I carry it along in this manner, and put a light to it, I think you will see it take fire, not with a bright light – you will at all events hear it, if you do not see it. Now, that is a body entirely different from oxygen: it is extremely light; for although yesterday you saw twice as much of this hydrogen produced on the one side as on the other, by the voltaic battery, it was only one-eighth the weight of the oxygen. I carry this jar upside-down. Why? Because I know that it is a very light body, and that it will continue in this jar upside-down quite as effectually as the water will in that jar which is not upside-down; and just as I can pour water from one vessel into another in the right position to receive it, so can I pour this gas from one jar into another when they are upside-down. See what I am about to do. There is no hydrogen in this jar at present, but I will gently turn this jar of hydrogen up under this other jar (fig. 28), and then we will examine the two. We shall see, on applying a light, that the hydrogen has left the jar in which it was at first, and has poured upwards into the other, and there we shall find it.
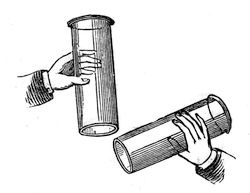
Fig. 28.
You now understand that we can have particles of very different kinds, and that they can have different bulks and weights; and there are two or three very interesting experiments which serve to illustrate this. For instance, if I blow soap bubbles with the breath from my mouth, you will see them fall, because I fill them with common air, and the water which forms the bubble carries it down. But now, if I inhale hydrogen gas into my lungs (it does no harm to the lungs, although it does no good to them), see what happens. [The Lecturer inhaled some hydrogen, and after one or two ineffectual attempts, succeeded in blowing a splendid bubble, which rose majestically and slowly to the ceiling of the theatre, where it burst.] That shews you very well how light a substance this is; for, notwithstanding all the heavy bad air from my lungs, and the weight of the bubble, you saw how it was carried up. I want you now to consider this phenomenon of weight as indicating how exceedingly different particles are one from the other; and I will take as illustrations these very common things – air, water, the heaviest body, platinum, and this gas: and observe how they differ in this respect; for if I take a piece of platinum of that size (fig. 29), it is equal to the weight of portions of water, air, and hydrogen of the bulks I have represented in these spheres. And this illustration gives you a very good idea of the extraordinary difference with regard to the gravity of the articles having this enormous difference in bulk. [The following tabular statement having reference to this illustration appeared on the diagram board.]
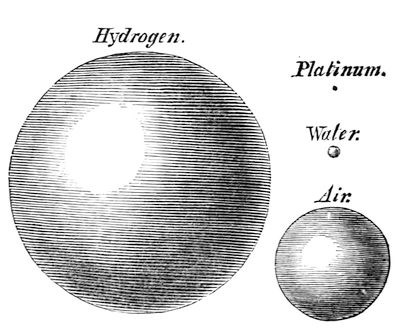
Fig. 29
Whenever oxygen and hydrogen unite together they produce water; and you have seen the extraordinary difference between the bulk and appearance of the water so produced, and the particles of which it consists chemically. Now, we have never yet been able to reduce either oxygen or hydrogen to the liquid state; and yet their first impulse, when chemically combined, is to take up first this liquid condition, and then the solid condition. We never combine these different particles together without producing water; and it is curious to think how often you must have made the experiment of combining oxygen and hydrogen to form water without knowing it. Take a candle, for instance, and a clean silver spoon (or a piece of clean tin will do), and if you hold it over the flame, you immediately cover it with dew – not a smoke – which presently evaporates. This perhaps will serve to shew it better. Mr. Anderson will put a candle under that jar, and you will see how soon the water is produced (fig. 30). Look at that dimness on the sides of the glass, which will soon produce drops, and trickle down into the plate. Well, that dimness and these drops are water, formed by the union of the oxygen of the air with the hydrogen existing in the wax of which that candle is formed.
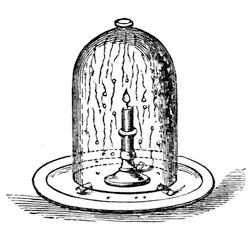
Fig. 30.
And now, having brought you in the first place to the consideration of chemical attraction, I must enlarge your ideas so as to include all substances which have this attraction for each other – for it changes the character of bodies, and alters them in this way and that way in the most extraordinary manner, and produces other phenomena wonderful to think about. Here is some chlorate of potash, and there some sulphuret of antimony.18 We will mix these two different sets of particles together; and I want to shew you in a general sort of way some of the phenomena which take place when we make different particles act together. Now, I can make these bodies act upon each other in several ways. In this case I am going to apply heat to the mixture; but if I were to give a blow with a hammer, the same result would follow. [A lighted match was brought to the mixture, which immediately exploded with a sudden flash, evolving a dense white smoke.] There you see the result of the action of chemical affinity overcoming the attraction of cohesion of the particles. Again, here is a little sugar19, quite a different substance from the black sulphuret of antimony, and you shall see what takes place when we put the two together. [The mixture was touched with sulphuric acid, when it took fire and burnt gradually, and with a brighter flame than in the former instance.] Observe this chemical affinity travelling about the mass, and setting it on fire, and throwing it into such wonderful agitation!
I must now come to a few circumstances which require careful consideration. We have already examined one of the effects of this chemical affinity; but to make the matter more clear we must point out some others. And here are two salts dissolved in water20. They are both colourless solutions, and in these glasses you cannot see any difference between them. But if I mix them, I shall have chemical attraction take place. I will pour the two together into this glass, and you will at once see, I have no doubt, a certain amount of change. Look, they are already becoming milky, but they are sluggish in their action – not quick as the others were – for we have endless varieties of rapidity in chemical action. Now, if I mix them together, and stir them, so as to bring them properly together, you will soon see what a different result is produced. As I mix them, they get thicker and thicker, and you see the liquid is hardening and stiffening, and before long I shall have it quite hard; and before the end of the lecture it will be a solid stone – a wet stone, no doubt, but more or less solid – in consequence of the chemical affinity. Is not this changing two liquids into a solid body a wonderful manifestation of chemical affinity?

