 Полная версия
Полная версияOn the various forces of nature and their relations to each other
Here is a piece of glass [producing a piece of plate-glass about two inches square] – (I shall want this afterwards to look to and examine its internal condition) – and here is some of the same sort of glass differing only in its power of cohesion, because while yet melted it has been dropped into cold water [exhibiting a “Prince Rupert’s drop”.10 (fig. 13)]; and if I take one of these little tear-like pieces and break off ever so little from the point, the whole will at once burst and fall to pieces. I will now break off a piece of this. [The Lecturer nipped off a small piece from the end of one of the Rupert’s drops, whereupon the whole immediately fell to pieces.] There! you see the solid glass has suddenly become powder – and more than that, it has knocked a hole in the glass vessel in which it was held. I can shew the effect better in this bottle of water; and it is very likely the whole bottle will go. [A 6-oz. vial was filled with water, and a Rupert’s drop placed in it, with the point of the tail just projecting out; upon breaking the tip off, the drop burst, and the shock being transmitted through the water to the sides of the bottle, shattered the latter to pieces.]
Here is another form of the same kind of experiment. I have here some more glass which has not been annealed [showing some thick glass vessels11 (fig. 14)], and if I take one of these glass vessels and drop a piece of pounded glass into it (or I will take some of these small pieces of rock crystal – they have the advantage of being harder than glass), and so make the least scratch upon the inside, the whole bottle will break to pieces, – it cannot hold together. [The Lecturer here dropped a small fragment of rock crystal into one of these glass vessels, when the bottom immediately came out and fell upon the plate.] There! it goes through, just as it would through a sieve.
Fig. 13. and Fig. 14.
Now, I have shewn you these things for the purpose of bringing your minds to see that bodies are not merely held together by this power of cohesion, but that they are held together in very curious ways. And suppose I take some things that are held together by this force, and examine them more minutely. I will first take a bit of glass, and if I give it a blow with a hammer, I shall just break it to pieces. You saw how it was in the case of the flint when I broke the piece off; a piece of a similar kind would come off, just as you would expect; and if I were to break it up still more, it would be as you have seen, simply a collection of small particles of no definite shape or form. But supposing I take some other thing, this stone for instance (fig. 15) [taking a piece of mica12], and if I hammer this stone, I may batter it a great deal before I can break it up. I may even bend it without breaking it; that is to say, I may bend it in one particular direction without breaking it much, although I feel in my hands that I am doing it some injury. But now, if I take it by the edges, I find that it breaks up into leaf after leaf in a most extraordinary manner. Why should it break up like that? Not because all stones do, or all crystals; for there is some salt (fig. 16) – you know what common salt is13: here is a piece of this salt which by natural circumstances has had its particles so brought together that they have been allowed free opportunity of combining or coalescing; and you shall see what happens if I take this piece of salt and break it. It does not break as flint did, or as the mica did, but with a clean sharp angle and exact surfaces, beautiful and glittering as diamonds [breaking it by gentle blows with a hammer]; there is a square prism which I may break up into a square cube. You see these fragments are all square – one side may be longer than the other, but they will only split up so as to form square or oblong pieces with cubical sides. Now, I go a little further, and I find another stone (fig. 17) [Iceland, or calc-spar]14, which I may break in a similar way, but not with the same result. Here is a piece which I have broken off, and you see there are plain surfaces perfectly regular with respect to each other; but it is not cubical – it is what we call a rhomboid. It still breaks in three directions most beautifully and regularly, with polished surfaces, but with sloping sides, not like the salt. Why not? It is very manifest that this is owing to the attraction of the particles, one for the other, being less in the direction in which they give way than in other directions. I have on the table before me a number of little bits of calcareous spar, and I recommend each of you to take a piece home, and then you can take a knife and try to divide it in the direction of any of the surfaces already existing. You will be able to do it at once; but if you try to cut it across the crystals, you cannot – by hammering, you may bruise and break it up – but you can only divide it into these beautiful little rhomboids.
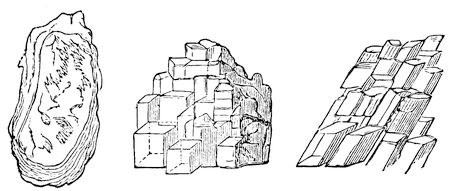
Fig. 15., Fig. 16. and Fig. 17.
Now I want you to understand a little more how this is – and for this purpose I am going to use the electric light again. You see, we cannot look into the middle of a body like this piece of glass. We perceive the outside form, and the inside form, and we look through it; but we cannot well find out how these forms become so: and I want you, therefore, to take a lesson in the way in which we use a ray of light for the purpose of seeing what is in the interior of bodies. Light is a thing which is, so to say, attracted by every substance that gravitates (and we do not know anything that does not). All matter affects light more or less by what we may consider as a kind of attraction, and I have arranged (fig. 18) a very simple experiment upon the floor of the room for the purpose of illustrating this. I have put into that basin a few things which those who are in the body of the theatre will not be able to see, and I am going to make use of this power, which matter possesses, of attracting a ray of light. If Mr. Anderson pours some water, gently and steadily, into the basin, the water will attract the rays of light downwards, and the piece of silver and the sealing-wax will appear to rise up into the sight of those who were before not high enough to see over the side of the basin to its bottom. [Mr. Anderson here poured water into the basin, and upon the Lecturer asking whether any body could see the silver and sealing-wax, he was answered by a general affirmative.] Now, I suppose that everybody can see that they are not at all disturbed, whilst from the way they appear to have risen up, you would imagine the bottom of the basin and the articles in it were two inches thick, although they are only one of our small silver dishes and a piece of sealing-wax which I have put there. The light which now goes to you from that piece of silver was obstructed by the edge of the basin, when there was no water there, and you were unable to see anything of it; but when we poured in water, the rays were attracted down by it, over the edge of the basin, and you were thus enabled to see the articles at the bottom.
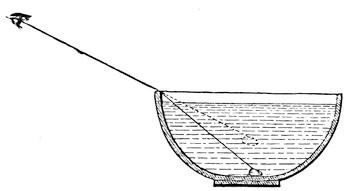
Fig. 18.
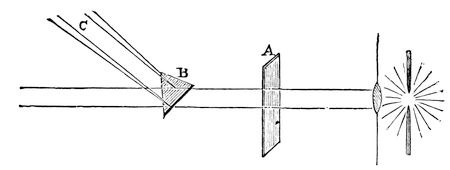
Fig. 19.
I have shewn you this experiment first, so that you might understand how glass attracts light, and might then see how other substances, like rock-salt and calcareous spar, mica, and other stones, would affect the light; and, if Dr. Tyndall will be good enough to let us use his light again, we will first of all shew you how it may be bent by a piece of glass (fig. 19). [The electric lamp was again lit, and the beam of parallel rays of light which it emitted was bent about and decomposed by means of the prism.] Now, here you see, if I send the light through this piece of plain glass, A, it goes straight through, without being bent, unless the glass be held obliquely, and then the phenomenon becomes more complicated; but if I take this piece of glass, B [a prism], you see it will shew a very different effect. It no longer goes to that wall, but it is bent to this screen, C; and how much more beautiful it is now [throwing the prismatic spectrum on the screen]. This ray of light is bent out of its course by the attraction of the glass upon it. And you see I can turn and twist the rays to and fro, in different parts of the room, just as I please. Now it goes there, now here. [The Lecturer projected the prismatic spectrum about the theatre.] Here I have the rays once more bent on to the screen, and you see how wonderfully and beautifully that piece of glass not only bends the light by virtue of its attraction, but actually splits it up into different colours. Now, I want you to understand that this piece of glass [the prism] being perfectly uniform in its internal structure, tells us about the action of these other bodies which are not uniform – which do not merely cohere, but also have within them, in different parts, different degrees of cohesion, and thus attract and bend the light with varying powers. We will now let the light pass through one or two of these things which I just now shewed you broke so curiously; and, first of all, I will take a piece of mica. Here, you see, is our ray of light. We have first to make it what we call polarised; but about that you need not trouble yourselves – it is only to make our illustration more clear. Here, then, we have our polarised ray of light, and I can so adjust it as to make the screen upon which it is shining either light or dark, although I have nothing in the course of this ray of light but what is perfectly transparent [turning the analyser round]. I will now make it so that it is quite dark; and we will, in the first instance, put a piece of common glass into the polarised ray, so as to shew you that it does not enable the light to get through. You see the screen remains dark. The glass then, internally, has no effect upon the light. [The glass was removed, and a piece of mica introduced.] Now, there is the mica which we split up so curiously into leaf after leaf, and see how that enables the light to pass through to the screen, and how, as Dr. Tyndall turns it round in his hand, you have those different colours, pink, and purple, and green, coming and going most beautifully – not that the mica is more transparent than the glass, but because of the different manner in which its particles are arranged by the force of cohesion.
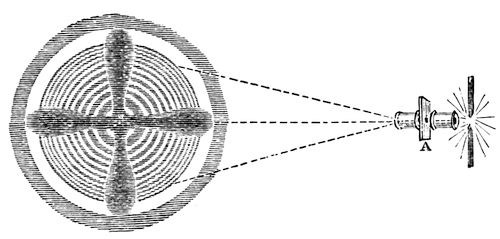
Fig. 20.
Now we will see how calcareous spar acts upon this light, – that stone which split up into rhombs, and of which you are each of you going to take a little piece home. [The mica was removed, and a piece of calc-spar introduced at A.] See how that turns the light round and round, and produces these rings and that black cross (fig. 20). Look at those colours – are they not most beautiful for you and for me? – for I enjoy these things as much as you do. In what a wonderful manner they open out to us the internal arrangement of the particles of this calcareous spar by the force of cohesion.
And now I will shew you another experiment. Here is that piece of glass which before had no action upon the light. You shall see what it will do when we apply pressure to it. Here, then, we have our ray of polarised light, and I will first of all shew you that the glass has no effect upon it in its ordinary state, – when I place it in the course of the light, the screen still remains dark. Now, Dr. Tyndall will press that bit of glass between three little points, one point against two, so as to bring a strain upon the parts, and you will see what a curious effect that has. [Upon the screen two white dots gradually appeared.] Ah! these points shew the position of the strain – in these parts the force of cohesion is being exerted in a different degree to what it is in the other parts, and hence it allows the light to pass through. How beautiful that is – how it makes the light come through some parts, and leaves it dark in others, and all because we weaken the force of cohesion between particle and particle. Whether you have this mechanical power of straining, or whether we take other means, we get the same result; and, indeed, I will shew you by another experiment that if we heat the glass in one part, it will alter its internal structure, and produce a similar effect. Here is a piece of common glass, and if I insert this in the path of the polarised ray, I believe it will do nothing. There is the common glass [introducing it] – no light passes through – the screen remains quite dark; but I am going to warm this glass in the lamp, and you know yourselves that when you pour warm water upon glass you put a strain upon it sufficient to break it sometimes – something like there was in the case of the Prince Rupert’s drops. [The glass was warmed in the spirit-lamp, and again placed across the ray of light.] Now you see how beautifully the light goes through those parts which are hot, making dark and light lines just as the crystal did, and all because of the alteration I have effected in its internal condition; for these dark and light parts are a proof of the presence of forces acting and dragging in different directions within the solid mass.
LECTURE III.
COHESION – CHEMICAL AFFINITY
We will first return for a few minutes to one of the experiments made yesterday. You remember what we put together on that occasion – powdered alum and warm water; here is one of the basins then used. Nothing has been done to it since; but you will find on examining it, that it no longer contains any powder, but a multitude of beautiful crystals. Here also are the pieces of coke which I put into the other basin – they have a fine mass of crystals about them. That other basin I will leave as it is. I will not pour the water from it, because it will shew you that the particles of alum have done something more than merely crystallise together. They have pushed the dirty matter from them, laying it around the outside or outer edge of the lower crystals – squeezed out as it were by the strong attraction which the particles of alum have for each other.
And now for another experiment. We have already gained a knowledge of the manner in which the particles of bodies – of solid bodies – attract each other, and we have learnt that it makes calcareous spar, alum, and so forth, crystallise in these regular forms. Now, let me gradually lead your minds to a knowledge of the means we possess of making this attraction alter a little in its force; either of increasing, or diminishing, or apparently of destroying it altogether. I will take this piece of iron [a rod of iron about two feet long, and a quarter of an inch in diameter], it has at present a great deal of strength, due to its attraction of cohesion; but if Mr. Anderson will make part of this red-hot in the fire, we shall then find that it will become soft, just as sealing-wax will when heated, and we shall also find that the more it is heated the softer it becomes. Ah! but what does soft mean? Why, that the attraction between the particles is so weakened that it is no longer sufficient to resist the power we bring to bear upon it. [Mr. Anderson handed to the Lecturer the iron rod, with one end red-hot, which he shewed could be easily twisted about with a pair of pliers.] You see, I now find no difficulty in bending this end about as I like; whereas I cannot bend the cold part at all. And you know how the smith takes a piece of iron and heats it, in order to render it soft for his purpose: he acts upon our principle of lessening the adhesion of the particles, although he is not exactly acquainted with the terms by which we express it.
And now we have another point to examine; and this water is again a very good substance to take as an illustration (as philosophers we call it all water, even though it be in the form of ice or steam). Why is this water hard? [pointing to a block of ice] because the attraction of the particles to each other is sufficient to make them retain their places in opposition to force applied to it. But what happens when we make the ice warm? Why, in that case we diminish to such a large extent the power of attraction that the solid substance is destroyed altogether. Let me illustrate this: I will take a red-hot ball of iron [Mr. Anderson, by means of a pair of tongs, handed to the Lecturer a red-hot ball of iron, about two inches in diameter], because it will serve as a convenient source of heat [placing the red-hot iron in the centre of the block of ice]. You see I am now melting the ice where the iron touches it. You see the iron sinking into it, and while part of the solid water is becoming liquid, the heat of the ball is rapidly going off. A certain part of the water is actually rising in steam – the attraction of some of the particles is so much diminished that they cannot even hold together in the liquid form, but escape as vapour. At the same time, you see I cannot melt all this ice by the heat contained in this ball. In the course of a very short time I shall find it will have become quite cold.
Here is the water which we have produced by destroying some of the attraction which existed between the particles of the ice, – for below a certain temperature the particles of water increase in their mutual attraction, and become ice; and above a certain temperature the attraction decreases, and the water becomes steam. And exactly the same thing happens with platinum, and nearly every substance in nature; if the temperature is increased to a certain point, it becomes liquid, and a further increase converts it into a gas. Is it not a glorious thing for us to look at the sea, the rivers, and so forth, and to know that this same body in the northern regions is all solid ice and icebergs, while here, in a warmer climate, it has its attraction of cohesion so much diminished as to be liquid water. Well, in diminishing this force of attraction between the particles of ice, we made use of another force, namely, that of heat; and I want you now to understand that this force of heat is always concerned when water passes from the solid to the liquid state. If I melt ice in other ways, I cannot do without heat (for we have the means of making ice liquid without heat; that is to say, without using heat as a direct cause). Suppose, for illustration, I make a vessel out of this piece of tinfoil [bending the foil up into the shape of a dish]. I am making it metallic, because I want the heat which I am about to deal with to pass readily through it; and I am going to pour a little water on this board, and then place the tin vessel on it. Now if I put some of this ice into the metal dish, and then proceed to make it liquid by any of the various means we have at our command, it still must take the necessary quantity of heat from something, and in this case it will take the heat from the tray, and from the water underneath, and from the other things round about. Well, a little salt added to the ice has the power of causing it to melt, and we shall very shortly see the mixture become quite fluid, and you will then find that the water beneath will be frozen – frozen, because it has been forced to give up that heat which is necessary to keep it in the liquid state, to the ice on becoming liquid. I remember once, when I was a boy, hearing of a trick in a country alehouse; the point was how to melt ice in a quart-pot by the fire, and freeze it to the stool. Well, the way they did it was this: they put some pounded ice in a pewter pot and added some salt to it, and the consequence was, that when the salt was mixed with it, the ice in the pot melted (they did not tell me anything about the salt, and they set the pot by the fire, just to make the result more mysterious), and in a short time the pot and the stool were frozen together, as we shall very shortly find it to be the case here. And all because salt has the power of lessening the attraction between the particles of ice. Here you see the tin dish is frozen to the board – I can even lift this little stool up by it.
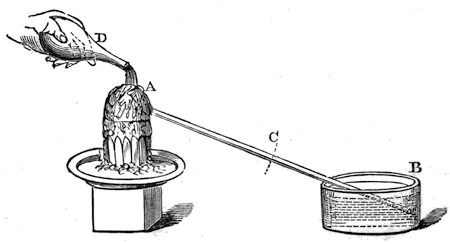
Fig. 21.
This experiment cannot, I think, fail to impress upon your minds the fact, that whenever a solid body loses some of that force of attraction by means of which it remains solid, heat is absorbed; and if, on the other hand, we convert a liquid into a solid, e. g., water into ice, a corresponding amount of heat is given out. I have an experiment shewing this to be the case. Here (fig. 21) is a bulb, A, filled with air, the tube from which dips into some coloured liquid in the vessel B. And I dare say you know that if I put my hand on the bulb A, and warm it, the coloured liquid which is now standing in the tube at C will travel forward. Now we have discovered a means, by great care and research into the properties of various bodies, of preparing a solution of a salt15 which, if shaken or disturbed, will at once become a solid; and as I explained to you just now (for what is true of water is true of every other liquid), by reason of its becoming solid, heat is evolved, and I can make this evident to you by pouring it over this bulb; – there! it is becoming solid, and look at the coloured liquid, how it is being driven down the tube, and how it is bubbling out through the water at the end; and so we learn this beautiful law of our philosophy, that whenever we diminish the attraction of cohesion, we absorb heat – and whenever we increase that attraction, heat is evolved. This, then, is a great step in advance, for you have learned a great deal in addition to the mere circumstance that particles attract each other. But you must not now suppose that because they are liquid they have lost their attraction of cohesion; for here is the fluid mercury, and if I pour it from one vessel into another, I find that it will form a stream from the bottle down to the glass – a continuous rod of fluid mercury, the particles of which have attraction sufficient to make them hold together all the way through the air down to the glass itself; and if I pour water quietly from a jug, I can cause it to run in a continuous stream in the same manner. Again, let me put a little water on this piece of plate-glass, and then take another plate of glass and put it on the water; there! the upper plate is quite free to move, gliding about on the lower one from side to side; and yet, if I take hold of the upper plate and lift it up straight, the cohesion is so great that the lower one is held up by it. See how it runs about as I move the upper one! and this is all owing to the strong attraction of the particles of the water. Let me shew you another experiment. If I take a little soap and water – not that the soap makes the particles of the water more adhesive one for the other but it certainly has the power of continuing in a better manner the attraction of the particles (and let me advise you, when about to experiment with soap-bubbles, to take care to have everything clean and soapy). I will now blow a bubble; and that I may be able to talk and blow a bubble too, I will take a plate with a little of the soapsuds in it, and will just soap the edges of the pipe, and blow a bubble on to the plate. Now, there is our bubble. Why does it hold together in this manner? Why, because the water of which it is composed has an attraction of particle for particle, – so great, indeed, that it gives to this bubble the very power of an india-rubber ball; for you see, if I introduce one end of this glass tube into the bubble, that it has the power of contracting so powerfully as to force enough air through the tube to blow out a light (fig. 22) – the light is blown out. And look! see how the bubble is disappearing, see how it is getting smaller and smaller.
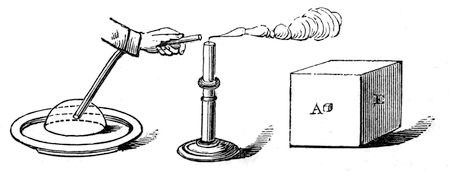
Fig. 22. and Fig. 23.
There are twenty other experiments I might shew you to illustrate this power of cohesion of the particles of liquids. For instance, what would you propose to me if, having lost the stopper out of this alcohol bottle, I should want to close it speedily with something near at hand. Well, a bit of paper would not do, but a piece of linen cloth would, or some of this cotton wool which I have here. I will put a tuft of it into the neck of the alcohol bottle, and you see, when I turn it upside down, that it is perfectly well stoppered, so far as the alcohol is concerned; the air can pass through, but the alcohol cannot. And if I were to take an oil vessel, this plan would do equally well, for in former times they used to send us oil from Italy in flasks stoppered only with cotton wool (at the present time the cotton is put in after the oil has arrived here, but formerly it used to be sent so stoppered). Now, if it were not for the particles of liquid cohering together, this alcohol would run out; and if I had time, I could have shewn you a vessel with the top, bottom, and sides altogether formed like a sieve, and yet it would hold water, owing to this cohesion.

