 Полная версия
Полная версияBuffon's Natural History. Volume X (of 10)
It is necessary to observe, that globes of clay heated white, lost more of their weight than iron bullets, even to the ninth or tenth part of their weight: whereas marl heated in the same fire, lost scarcely any thing, although the whole surface was covered over with scales, and reduced into glass. As this appeared singular, I repeated the experiment several times, increasing the fire, and continuing it longer than for iron; and although it scarcely required a third of the time to redden marl, to what it did to redden iron, I kept them in the fire thrice as long as was requisite, to see if they would lose more, but I found very trifling di minutions; for the globe of two inches heated for eight minutes, which weighed seven ounces, two drachms, and thirty grains, before it was put in the fire, lost only forty-one grains, which does not make a hundredth part of its weight; and that of three inches, which weighed twenty-four ounces, five drachms, and thirteen grains, having been heated by the fire for eighteen minutes, that is nearly as much as iron, lost only seventy-eight grains, which does not make the hundredth and eighty-first part of its weight. These losses are so trifling, that it may be looked upon, in general, as certain that pure clay loses nothing of its weight in the fire; for those trifling diminutions were certainly occasioned by the ferruginous parts which were found in the clay, and which were in part destroyed by the fire. It is also worthy of observation, that the duration of heat in different matters exposed to the same fire for an equal time, is always in the same proportion, whether the degree of heat be greater or smaller.
I have made similar experiments on globes of marble, stone, lead, and tin, by a heat only strong enough to melt tin, and I found, that iron refrigerated in eighteen minutes, so as to be able to hold it in the hand, marble refrigerated to the same degree in twelve minutes, stone in eleven, lead in nine, and tin in eight. It is not, therefore, in proportion to their density, as is commonly supposed, that bodies receive and lose more or less heat, but in an inverse ratio of their solidity; that is, of their greater or lesser non fluidity; so that, by the same heat, less time is requisite to heat or cool the most dense fluid.
To prevent the suspicion of vainly dwelling upon assertion, I think it necessary to remark upon what foundation I build this theory; I have found that bodies which should heat in ratio of their diameters, could be only those which were perfectly permeable to heat, and would heat or cool in the same time; hence, I concluded that fluids, whose parts are only held together by a slight connection, might approach nearer to this perfect permeability than solids, whose parts have more cohesion. In consequence of this, I made experiments, by which I found, that with the same heat all fluids, however dense they might be, heat and cool more readily than any solids, however light, so that mercury, for example, heats much more readily than wood, although it be fifteen or sixteen times more dense.
This made me perceive that the progress of heat in bodies cannot, in any case, be made relatively to their density; and I have found by experience, that this progress, as well in solids as fluids, is made rather by reason of their fluidity, or in an inverse ratio of their solidity. I mean by solidity the quality opposite to fluidity; and I say, that it is in an inverse ratio of this quality that the progress of heat is made in both bodies; and that they heat or cool so much the faster as they are the more fluid, and so much the slower as they are more solid, every other circumstance being equal.
To prove that solidity, taken in this sense, is perfectly independent of density, I have found, by experience, that the most or least dense matters, heat or cool more readily than other more or less dense matters, for example, gold or lead, which are much more dense than iron and copper, heat and cool much quicker; while tin and marble, which are not so dense, heat and cool much faster than iron and copper; and there are likewise many other matters which come under the same description; so that density is in no manner relative to the scale of the progress of heat in solid bodies.
It is likewise the same in fluids, for I have observed, that quicksilver, which is thirteen or fourteen times more dense than water, nevertheless heats and cools in less time than water; and spirit of wine, which is less dense than water, heats and cools much quicker; so that generally the progress of heat in bodies, as well with regard to the ingress as egress, has no affinity with their density, and is principally made in the ratio of their fluidity, by extending the fluidity to a solid; from hence I concluded, that we should know the real degree of fluidity in bodies, by heating them to the same heat; for their fluidity would be in a like ratio as that of the time during which they would receive and lose this heat; and that it would be the same with solid bodies. They will be so much the more solid, that is to say, so much the more non fluids, as they require more time to receive and lose this heat, and that almost generally to what I presume; for I have already tried these experiments on a great number of different matters, and from them I have made a table, which I have endeavoured to render as complete and exact as possible.
I caused several globes to be made of an inch diameter with the greatest possible precision, from the following matters, which nearly represent the Mineral kingdom.
M. Tillet, of the Academy of Sciences, made the globe of refined gold at my particular request, and the whole of them weighed as follows:
I must here observe, that a positive conclusion must not be made of the exact specific weight of each matter from the preceding table, for notwithstanding the precaution that was taken to render the globes equal, yet, as I was obliged to employ different workmen, some were too large, and others too small. Those which were more than an inch diameter were diminished, but those of rock chrystal, glass and porcelain, which were rather too small, we suffered to remain, and only rejected those of agate, jasper, and porphyry, which were sensibly so. This precision in size was however not absolutely necessary, for it could very little alter the result of my experiments.
Previously to ordering these globes, I exposed to a like degree of fire, a square mass of iron, and another of lead of two inches diameter, and found, by reiterated essays, that lead heated and cooled in much less time than iron. I made the same experiment on red copper, and that required more time to heat and cool than lead, and less than iron. So that of these three matters, iron appeared the least accessible to heat, and, at the same time, that which retained it the longest. From which I learn that the law of the progress of heat in bodies was not proportionable to their density, since lead, which is more dense than iron or copper, nevertheless heats and cools in less time than either. As this object appeared important, I was induced to have these globes made, and to be more perfectly satisfied of the progress of heat in a great number of different matters, I always placed the globes at an inch distance from each other, before the same fire, or in the same oven, 2, 3, 4, or 5, together with a globe of tin in the midst of them. In most of my experiments I suffered them to be exposed to the same active fire till the globe of tin began to melt, and at that instant they were all removed, and placed on a table in small cases. I suffered them to cool without moving, often trying whether I could touch them, and the moment they left off burning, and I could hold them in my hands half a second, I marked the time which had passed since I drew them from the fire. I afterwards suffered them to cool to the actual temperature, of which I endeavoured to judge by means of touching other small globes of the same matters that had not been heated. Of all the matters which I put to the trial, there was only sulphur which melted in a less degree of heat than tin, and notwithstanding its disagreeable smell I should have taken it for a term of comparison, but being a brittle matter which diminishes by friction, I preferred tin, although it required nearly double the heat to melt.
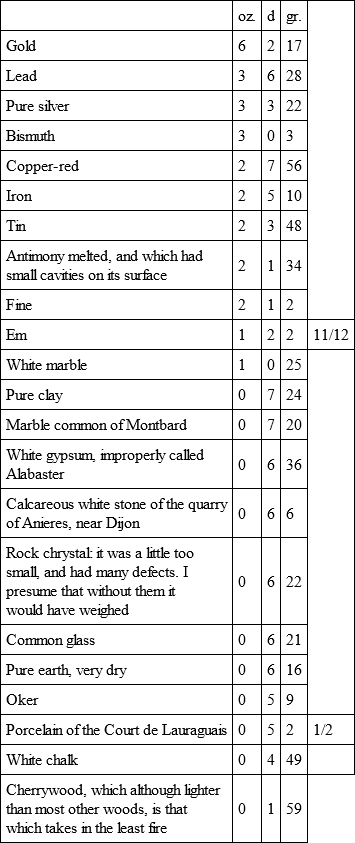
Having heated together bullets of iron, copper, lead, tin, gres, and Montbard marble, they cooled in the following order:
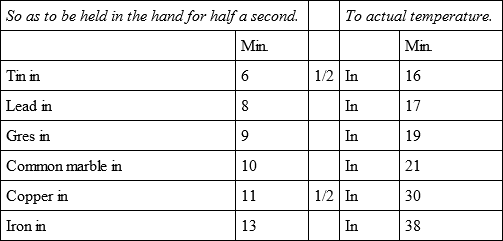
By a second experiment with a fiercer fire, sufficient to melt the tin bullet, the five others cooled.
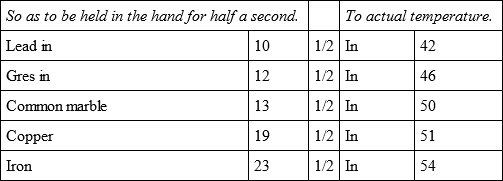
By a third experiment, with a less degree of fire than the preceding, the same bullets with a fresh tin bullet, cooled in the following manner.
From these experiments, which I made with as much precision as possible, we may conclude, first, that the time of refrigeration of iron, so as to be held in the hand, is to that of copper:: 531/2: 45, and so to the point of temperature:: 142: 125.
2dly, That the time of refrigeration of iron, so as to be held in the hand, is to that of the first refrigeration of common marble:: 531/2: 351/2 and their entire refrigeration:: 142: 110.
3dly, that the time of refrigeration of iron, to that of gres, so as to be held in the hand, is:: 531/2: 32 and:: 142: 1021/2, for their entire refrigeration.
4thly, That the time of refrigeration of iron to that of lead, so as to be held in the hand, is:: 531/2: 27 and 142: 941/2 for their entire refrigeration.
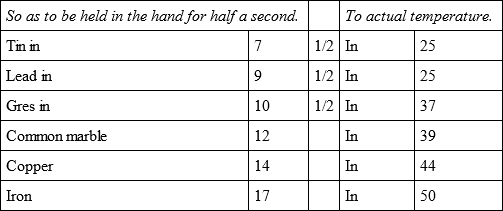
In an oven hot enough to melt tin, although all the coals and cinders were drawn out, I placed, on a piece of iron wire, five bullets, distant from one another about nine lines, after which the oven was shut, and having drawn them out, in about 18 minutes they cooled,
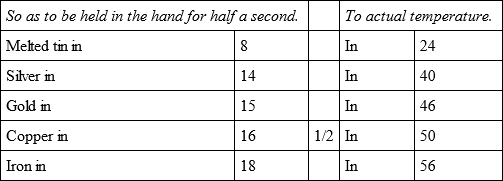
In the same oven, but with a slower heat, the same bullets with an other bullet of tin, cooled,
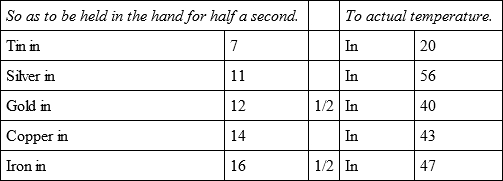
In the same oven, but with a still less degree of heat, the same bullets cooled,
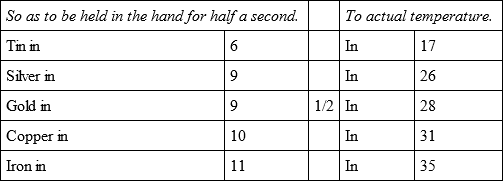
Having placed in the same oven five other bullets, placed the same and separated from each other, their refrigeration was in the following proportions.
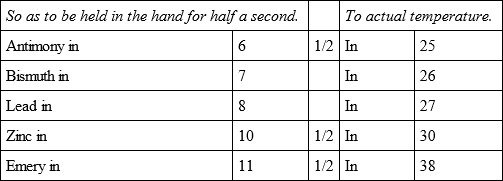
In the same oven, and in the same manner, another bullet of Bismuth was placed, with six other bullets, which cooled,
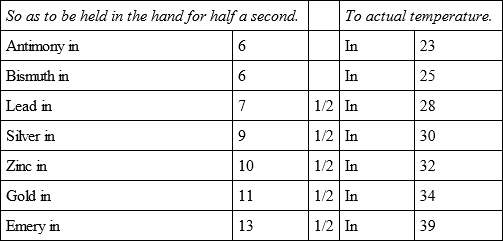
There was put in the same oven a bullet of glass, another of tin, one of copper, and one of iron, and they cooled, of iron, and they cooled,
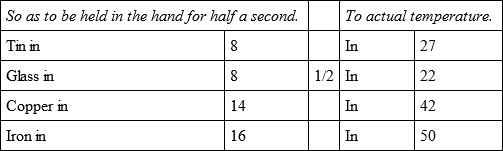
Bullets of gold, glass, porcelain, gypsum, and gres, were heated together, and cooled,
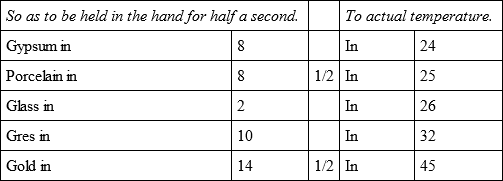
Bullets of silver, common marble, hard stone, white marble, and soft calcareous stone of Anieres, near Dijon, were heated like the former, and cooled,
The whole of these experiments were made with the utmost care and attention, not only by myself but in the presence of several persons, who also endeavoured to judge of the first degree of temperature by holding the bullets for half a second in their hands, and the relations of which are more exact than those of the actual temperature, because that being variable the result must sometimes vary also.
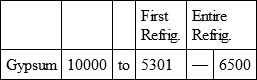
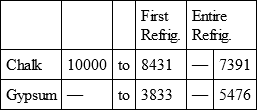
With a view to avoid that prolixity which would necessarily attend the continual repetition in a comparative statement of the refrigeration of these different bodies, we have connected them in a general table, and taking 10,000 for the standard of comparison, their differences may be seen at one view.
A TABLE
OF THE
Relations of different Mineral Substances
IRON, with
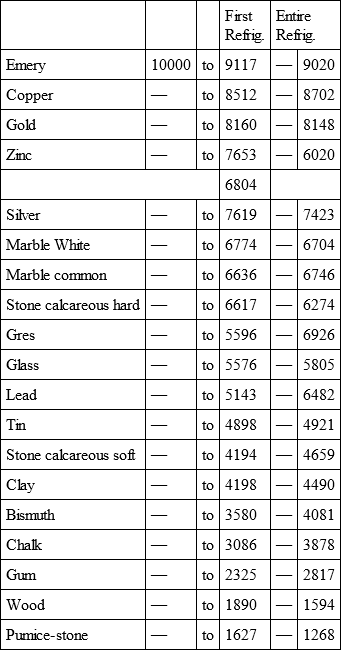
EMERY, with
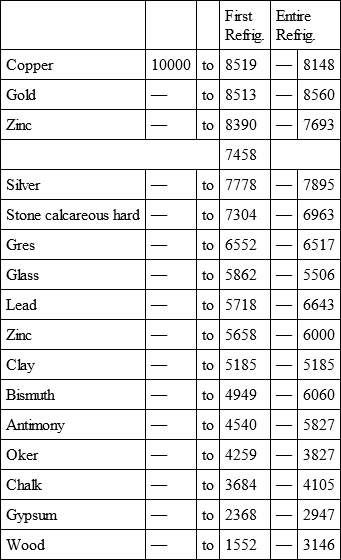
COPPER, with
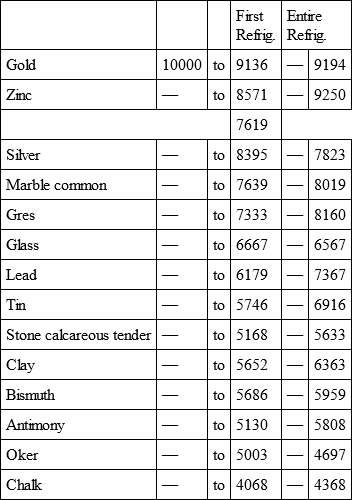
GOLD, with
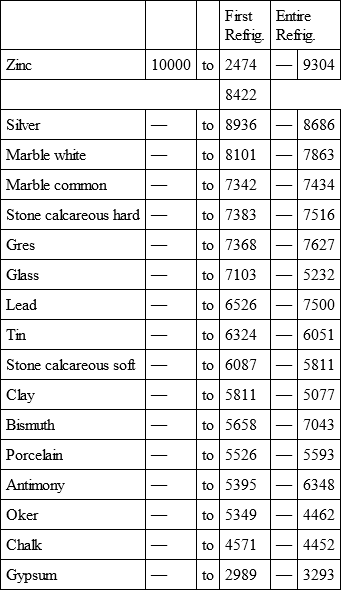
ZINC, with
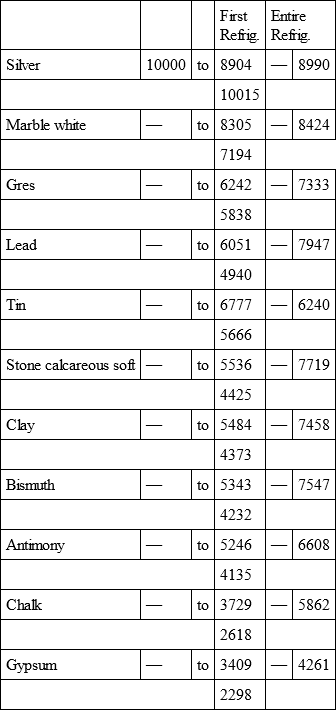
SILVER, with
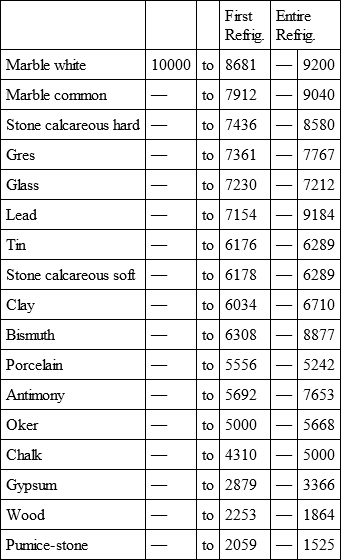
WHITE MARBLE, with
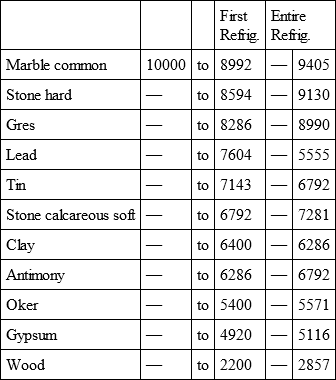
COMMON MARBLE, with
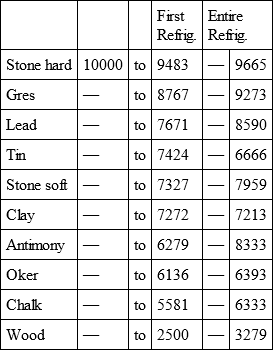
HARD CALCAREOUS STONE, with
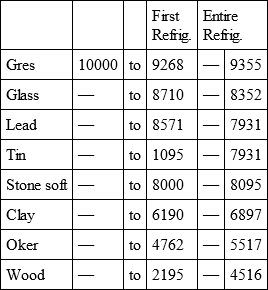
GRES, with
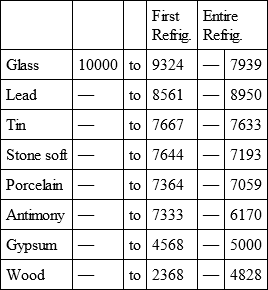
GLASS, with
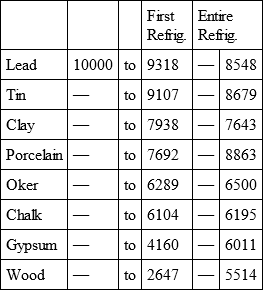
LEAD, with
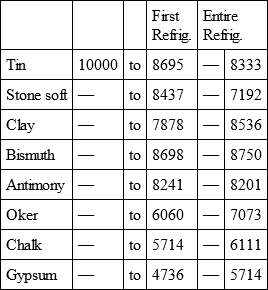
TIN, with
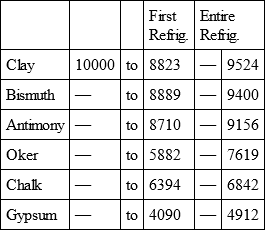
STONE CALCAREOUS SOFT, with
CLAY, with
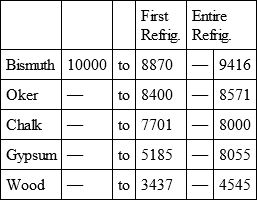
BISMUTH, with
PORCELAIN, with
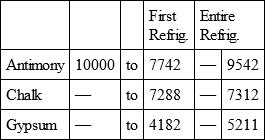
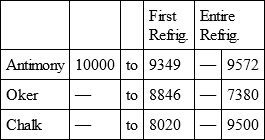
ANTIMONY, with
OKER, with
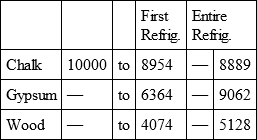
CHALK, with
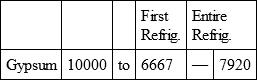
GYPSUM, with
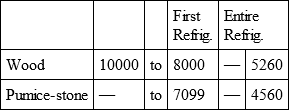
WOOD, with
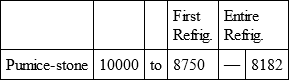
Notwithstanding the assiduity I used in my experiments, and the care I took to render the relations exact, I own there are still some imperfections in the foregoing table; but the defects are trivial, and do not much influence the general results; for example, it will easily be perceived, that the relation of zinc to lead being 10,000 to 6,051, that of zinc to tin should be less than 6,000, whereas it is found 6,777 in the table. It is the same with respect of silver to bismuth, which ought to be less than 6,308, and also with regard of lead to clay, which ought to be more than 8,000, but in the table is only 7,878. This difference proceeded from the leaden and bismuth bullets not being always the same; they melted, as well as those of tin and antimony, and, therefore, could not fail to produce variations, the greatest of which are the three I have just remarked. It was not possible for me to do better; the different bullets of lead, tin, bismuth, and antimony, which I successively made use of, were made in the same manner, but the matter of each might be somewhat different, according to the quantity of the alloy in the lead and tin, for I had pure tin only for the two first bullets; besides, there remains very often a small cavity in the melted bullet, and these little causes are sufficient to produce the little differences which may be remarked in the table.
On the whole, to draw from these experiments all the profit that can be expected, the matters which compose their object must be divided into four classes, viz. 1. Metals. 2. Semi-metals and Metallic Minerals. 3. Vitreous and Vitrescible Substances. And 4. Calcareous and Calcinable substances. Afterwards the matters of each class must be compared between themselves to discover the cause, or causes, or the order which follows the progress of heat in each, and then with each other, in order to deduce some general results.
First. The order of the six metals, according to their density, is tin, iron, copper, silver, lead, and gold; whereas the order in which they receive and lose their heat is tin, lead, silver, gold, copper, and iron; so that in tin alone it retains its place.
The progress and duration of heat in metals does not then follow the order of their density, except in tin, which being the least dense, is also that which soonest loses its heat; but the order of the five other metals demonstrates that it is in relation to their fusibility that they all receive and loose heat; for iron is more difficult to melt than copper, copper more than gold, gold more than silver, silver more than lead, lead more than tin; and therefore we may conclude that it is only by chance if the density and fusibility of tin be found so united as to place it in the last rank. Nevertheless, it would be advancing too much to pretend that we must attribute all to fusibility, and nothing to density. Nature never deprives herself of one of her properties in favour of another in an absolute manner; that is to say, in a mode that the first has not any influence on the second. Thus, density may be of some weight in the progress of heat; but we may safely affirm, that in the six metals it has very little comparatively with fusibility.
This fact was neither known to chemists nor naturalists; they did not even imagine that gold which is more than twice as dense as iron, nevertheless loses its heat near a third sooner. It is the same with lead, silver, and copper, which are all more dense than iron, and which, like gold, heat and cool more readily; for though the object of this, second memoir was only refrigeration, yet the experiments of the one that preceded it demonstrate, that there is ingress and egress of heat in bodies, and that those which receive it most quickly also lose it the soonest.
If we reflect on the real principles of density, and the cause of fusibility, we shall perceive, that density depends absolutely on the quantity of matter which Nature places in a given space; that the more she can make it enter therein, the more density there will be, and that gold, in this respect, is of all substances, that which contains the most matter relatively to its volume. It is for this reason that it has been hitherto thought, that more time is required to heat or cool gold than other metals; and it is natural enough to suppose, that containing double or treble the matter in the same volume, double or treble time would be required to penetrate it with heat; nay this would be true, if in every substance the constituent parts were of the same figure and ranged the same. But in the most dense the molecules of matter are, probably, of a figure sufficiently regular not to leave very void places between them; in others which are not so dense, and their figures more irregular, more vacuities are left, and in the lightest, the molecules being few, and most likely of a very irregular figure, a thousand times more void is found than plenitude; for it may be demonstrated by other experiments, that the volume of even the most dense substance contains more void space than full matter.
Now, the principal cause of fusibility is the facility which the particles of heat find in separating these molecules of full matter from each other; let the sum of the vacuities be greater or less, which causes density or lightness, it is indifferent to the separation of the molecules which constitute the plenitude; and the greater or less fusibility depends entirely on the power of coherence which retains the massive parts united, and opposes itself more or less to their separation. The dilatation of the total volume is the first degree of the action of heat; and in different metals it is made in the same order as the fusion of the mass, which is performed by a greater degree of heat or fire. Tin, which melts the most readily, is also that which dilates the quickest; and iron, which is the most difficult of all to melt, is likewise that whose dilatation is the slowest.
After these general positions, which appear clear, precise, and founded on experiments that nothing can contradict, it might be imagined that ductility would follow the order of fusibility, because the greater or less ductility seems to depend on the greater or less adhesion of the parts in each metal; nevertheless, ductility seems to have as much connection with the order of density, as with that of their fusibility. I would even affirm that it is in a ratio composed of the two others, but that would be only by estimation, and a presumption which is, perhaps not founded; for it is not so easy to exactly determine the different degrees of fusibility, as those of density; and as ductility participates of both, and varies according to circumstances, we have not as yet acquired the necessary knowledge to pronounce affirmatively on this subject, though it is most certainly of sufficient importance to merit particular researches. The same metal when cold gives very different results to what it does when hot, although treated in the same manner. Malleability is the first mark of ductility; but that gives only an imperfect idea of the point to which ductility may extend; nor can simple lead, the most malleable metal, be drawn into such fine threads as gold, or even as iron, which is the least malleable. Besides we must assist the ductility of metals with the addition of fire, without which they become brittle: even iron, although the most robust, is brittle like the rest. Thus the ductility of one metal, and the extent of continuity which it can support, depend not only on its density and fusibility, but also on the manner and space in which it is treated, and of the addition of heat or fire which is properly given to it.
II. By comparing those substances which we term semi-metals and metallic minerals, which want ductility, we shall perceive, that the order of their density is emery, zinc, antimony and bismuth, and that in which they receive and lose heat, is antimony, bismuth, zinc, and emery; and which does not in any measure follow the order of their density, but rather that of their fusibility. Emery, which is a ferruginous mineral, although as dense again as bismuth, retains heat longer. Zinc, which is lighter than antimony or bismuth, retains heat longer than either. Antimony and bismuth, receive and keep it nearly alike. There are, therefore, semi-metals, and metallic minerals, which, like metals, receive and lose heat nearly in the same relation as their fusibility, and partake very little of their density.



