
Полная версия:
Gulls
FOOD AND FEEDING
Despite being small, the Black-headed Gull has been recorded feeding on a wide range of items, although in general these are smaller than those taken by the larger gulls. Animal material dominates, with invertebrates being by far the most frequently consumed food. Earthworms and the adult and larval stages of beetles and flies predominate in food obtained in fields. In coastal areas, marine crustaceans, molluscs and worms are frequently consumed when mudflats are exposed at low tide, or small food items are taken from the sea surface (Fig. 37). In addition to the consumption of animal matter, the gulls ingest a wide range of plant materials from time to time. Some of these are ingested incidentally with animal food, but seeds and grain are intentionally consumed – in autumn, for example, acorns are occasionally plucked from the tops of oak trees and Hawthorn (Crataegus monogyna) berries are picked off hedgerows by birds momentarily alighting on branches. A wide range of animal and vegetable materials, including bread, are taken around human habitation and at landfill sites. In some areas, bread and food scraps are regularly supplied by the public, particularly in parks and by lakes, and these form a major part of the winter diet in urban areas.
The gulls look for potential food either by flying 1–2 m over the ground or water and landing to pick up items, or (more frequently) by landing, usually as a dense flock in a field, where they spread out by walking over the ground. This latter habit is used extensively just after dawn, as soon as the gulls arrive from their night roosts and when earthworms are still active on or near the ground surface. Small fish and riparian insects are captured and consumed on or near freshwater sources. Insects such as swarming winged ants are caught in flight on calm days in late summer. At times, adults bring large quantities of hairy caterpillars collected from moorland to feed to their young, but the hairs of some of these species are irritants and many chicks regurgitate them in numbers, such that they sometimes litter the ground within the colony. Occasional small mammals and amphibians are consumed, but these form a minute part of the diet.

FIG 37. Black-headed Gulls feeding on small fish larvae. Note the unusual head position. (Norman Deans van Swelm)
Kleptoparasitism
Kleptoparasitism is common in Black-headed Gulls, whereby the birds steal from members of their own species and, particularly, flocks of Lapwings (Vanellus vanellus). Golden Plovers (Pluvialis apricaria) feeding on agricultural land in winter also regularly have their food stolen by the gulls. Lapwings and Golden Plovers feed mainly on earthworms and insect larvae obtained by actively searching the ground, and they are often joined by Black-headed Gulls (and sometimes Common Gulls, Larus canus). However, rather than searching for worms, some gulls just stand nearby or follow the plovers; when a plover finds a large food item that requires manipulation before it can be swallowed, it is rapidly and often successfully challenged by one of the gulls, and frequently the food item is stolen. In other cases, a plover may take off with the worm or insect in its bill, only to be closely pursued by a gull, which attempts to force it to drop the prey. When this happens, the gull takes the food as it falls or quickly turns to pick it up from the ground.
Such kleptoparasitism of Lapwings and Golden Plovers decreases the waders’ own rate of food consumption, but this is partially mitigated by feeding at night under full moon conditions when the gulls are absent. The fact that Black-headed Gulls resort to kleptoparasitism suggests that they are not as efficient as plovers at searching and finding their own earthworms.
Kleptoparasitism by Black-headed Gulls appears to be a specialised feeding behaviour used by a minority of individuals, and experience is important in successfully obtaining food in this way. Even by their second year of life, young Black-headed Gulls have still not achieved the efficiency of adults when carrying out kleptoparasitic attacks (Hesp & Barnard, 1989).
In urban areas in winter, the tables are often turned on Black-headed Gulls. Those birds finding large food items that they fail to swallow immediately are frequently pursued by Common Gulls or even members of their own species, until they are forced to drop the food to the benefit of the pursuers. Such pursuits are particularly frequent in urban areas during severe winter weather, when large numbers of Common Gulls move from snow-covered high ground and congregate at much increased densities at urban feeding sites.
Feeding areas
In the breeding season, the feeding areas and ranges of Black-headed Gulls are limited by the location of the colony, and well-defined flight lines are often evident to and from large colonies. Many feeding adults forage within 10 km of the colony, although some will travel 40 km or more to favoured sites with a regular supply of food provided through the activities of humans. These may be fields where ploughing is in progress, landfill sites, large car parks (particularly near food stores), coastal picnic sites or riverbanks in towns where people feed bread, chips and other items to birds. Food supplements put out for farm animals are also exploited. Few Black-headed Gulls hunt far from the shore at coastal sites, and only rarely do they plunge-dive and submerge to capture fish.
In the 1960s, along the 18 km tidal reaches of the river Tyne (which was little more than an open sewer at the time), Black-headed and Common gulls were the commonest birds feeding in winter at sewage outfalls and on sewage items floating in the river. Once the sewage was piped separately to treatment plants to clean the river up, the numbers of Common Gulls reduced dramatically, but Black-headed Gulls remained abundant and were still able to find food in the river. This is because the outflow of filtered water passing from sewage plants into the river still contained small items, which were attractive to the Black-headed Gulls but not the Common Gulls.
The habit of large numbers of Black-headed Gulls following tractors ploughing fields is both widespread and spectacular, with many individuals competing for position just behind the plough blades, and diving to catch and consume worms or insects exposed when the soil is turned over. Similar feeding occurs when grass is being cut for hay. The introduction of grass cutting earlier in the year for silage attracts many Black-headed Gulls and has had a marked effect in some areas where the birds feed. Although silage production was introduced many years ago, it became far more extensive from about 1969, and by 1993 it had increased sevenfold as a means of producing winter cattle feed. Grass is now frequently cut twice a year for silage and much earlier in the season than for hay. This has allowed feeding by Black-headed Gulls on insects in these fields to be spread over a much longer period of the species’ breeding season than was once the case, and presumably has been of benefit to the gulls.
Feeding at landfill sites in winter attracts flocks of hundreds and even thousands of Black-headed Gulls. It is a highly social activity and occurs at irregular intervals, often with a resting, inactive flock remaining nearby for long periods. From time to time, one or two individuals leaving the flock will then fly over the landfill working area and alight there or pick up a food item while in flight. This act of feeding by one or two individuals is a major stimulus to those birds in the resting flock, and the great majority will then leave the roost and stream over to the feeding site, land on the refuse and search for food – several hundred individuals may be highly active within a small area. Suddenly, feeding will stop, perhaps as the result of a loud noise, the approach of a person or the arrival of a lorry. On other occasions and for no apparent reason for alarm, the flock will rise as one and return to the roosting site, staying away from the landfill working area for many minutes or even hours.
Food-collecting techniques
Black-headed Gulls use several techniques when collecting food, summarised below:
1. Aerial searching Individuals patrol suitable areas, turning and swooping to pick up items discovered. Used on farmland and in urban areas.
2. Walking over fields of short grass The birds are usually spread out and search for food items on the ground.
3. Feeding while floating on water The birds swim buoyantly, turning rapidly to pick small items such as larval fish from the water’s surface, phalarope-like (Fig. 37). Head-dipping into the water is less frequent and diving does not take place.
4. Feeding frenzies Many individuals converge on an appreciable food source, such as food discarded by the public, a freshly ploughed field or refuse at a landfill site. In all three cases, individuals are attracted from some distance by the erratic flight and calling of those birds already scrambling for food.
5. Feeding on flying insects This is most frequent in autumn, when winged ants take to the air on calm, warm days. The gulls ‘hawk’ after the insects, flying slowly and frequently changing direction.
6. Consumption of items from trees, bushes and low plants This is an infrequent method, with gulls approaching branches or low plants, landing on them momentarily, and plucking abundant insects from the leaves and thin branches. Similar methods are used to obtain acorns from oak trees and berries from Hawthorn bushes.
7. Paddling in shallow pools on intertidal mud This technique is used to disturb, detect and capture small invertebrates, including marine worms. While Herring Gulls also use this method on areas of short grass, this has not been reported in Black-headed Gulls.
8. Kleptoparasitism The birds steal food from Lapwings and Golden Plovers when the waders are feeding on soil invertebrates in pastures.
Use of winter feeding areas
The next sections are based on data gathered through extensive ringing and wing-tagging studies of Black-headed Gulls wintering in north-east England. Observing coloured leg rings on Black-headed Gulls was difficult in resting flocks and when they were feeding on grasslands, so to overcome this problem, plastic wing tags were used (here). These proved more successful in identifying and following the behaviour of individuals throughout the day. The collaborative research involved identifying feeding areas and catching and marking birds prior to and during three years of intensive field studies on the species made, and was carried out by Gabriella MacKinnon at Durham University as part of her doctoral thesis (MacKinnon, 1986). It involved us in many cold early-morning starts in winter to reach the feeding areas and set cannon nets before the gulls arrived at about sunrise from their night roosts.
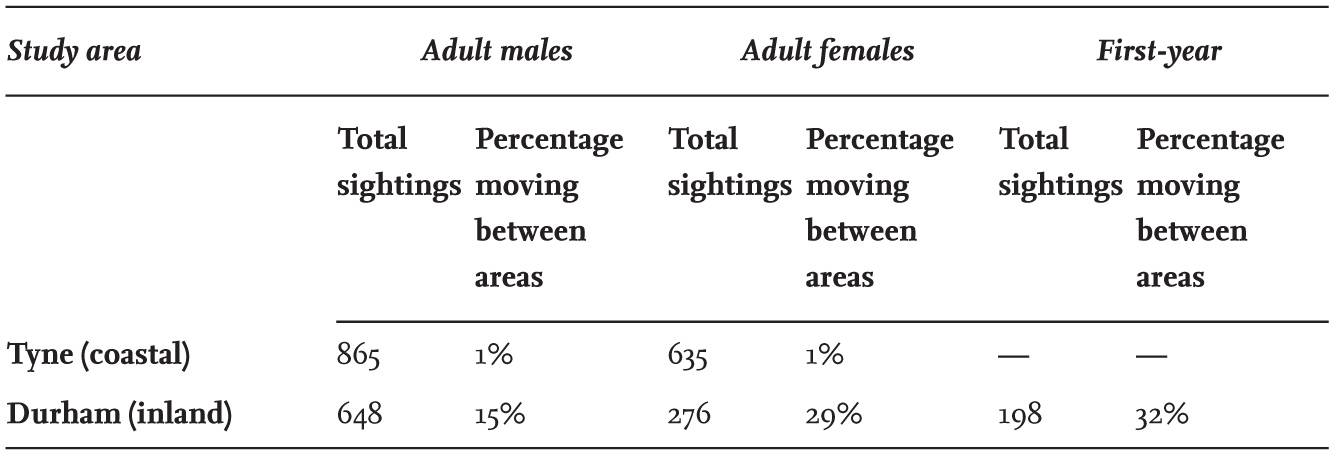
Most of the wing-tagged individuals studied in north-east England frequently returned to feed at the same sites during the whole winter (Table 14), but some moved away during this period. A marked difference in the behaviour of the gulls was detected between those using coastal sites and those feeding inland. At the coast, both adult males and females were particularly faithful to the same area throughout the winter, but at inland sites both sexes were less faithful, with adult females nearly twice as likely to move elsewhere than adult males. This difference between coastal and inland sites probably relates to the difficulty of accessing food in fields during periods of cold, frosty weather, which is less of a problem at coastal and intertidal areas as they are less susceptible to freezing. First-year birds were variable in the proportions that returned to feed at the same site, but overall, they were less faithful to sites than adults.
Sightings of wing-tagged birds that moved 10 km or more from the study areas in north-east England in the same winter and reported by other observers revealed that all moved south, remained on or near the east coast of England, and moved less than 100 km, apart from one bird, which moved more than 350 km. Of the 254 adults marked in winter in north-east England, 19 were seen in Britain during the breeding season. Nine of these birds were recorded within 50 km of the winter study areas, while 51 were recorded in colonies on the Continent, confirming that most of the marked birds studied bred outside Britain.
That some individuals return to the same feeding areas each day is remarkable – in some cases during the field studies, birds commuted up to 25 km twice a day to and from a large overnight roost on land containing at least 10,000 birds. Observations of the Black-headed Gulls leaving their night roost in the early morning revealed that individuals from several parts of the roost left in successive waves spread over the 30 minutes before sunrise. Some of those leaving from different parts of the roost circled, formed flocks and departed inland. The birds arriving at sunrise at an extensive series of sport fields in Durham 25 km away gathered in one or two flocks, and invariably included several wing-tagged birds that were previously marked when feeding there. Yet some of these tagged birds (and probably the others) did not roost close to each other at night. How individuals dispersing from the night roost among thousands of others managed to gather into a flock returning to the same feeding grounds is a fascinating mystery to which I have no answer. How do birds regularly flying to the same feeding area manage to group together into a flock on leaving the large roost? This is an interesting aspect of behaviour that requires more research, which might be assisted through the use of small modern transmitters (here).
TABLE 14. The proportion of wing-tagged Black-headed Gulls in north-east England recorded in subsequent weeks during the same winter in a different feeding area. Data summarised from MacKinnon (1986).
Age structure in different feeding areas
First-year and adult Black-headed Gulls are not distributed in the same proportions in different feeding habitats within the same area. During the studies carried out in north-east England, first-year birds formed only 1–5 per cent of the Black-headed Gulls present near river mouths and on the coast (Table 15), and were more than four times as frequent feeding inland on pastures and grassland, averaging 22 per cent of individuals present. At landfills, first-year birds increased from 11 per cent in autumn to 20 per cent by midwinter. The reason for these differences is not known, but since birds feeding on the coast and those feeding inland used the same night roost, it would appear to be the result of variation in the feeding ability of birds of different ages (Table 16). This suggests that coastal feeding may require greater experience in order to become proficient, as has been shown in other studies of Black-headed Gulls relying on kleptoparasitism as a source of food (here).
TABLE 15. The proportion of first-year Black-headed Gulls in winter counts near the mouths of four rivers in north-east and eastern England, and on the north-east coast. Proportions of first-year birds showed little variation between days or between years.
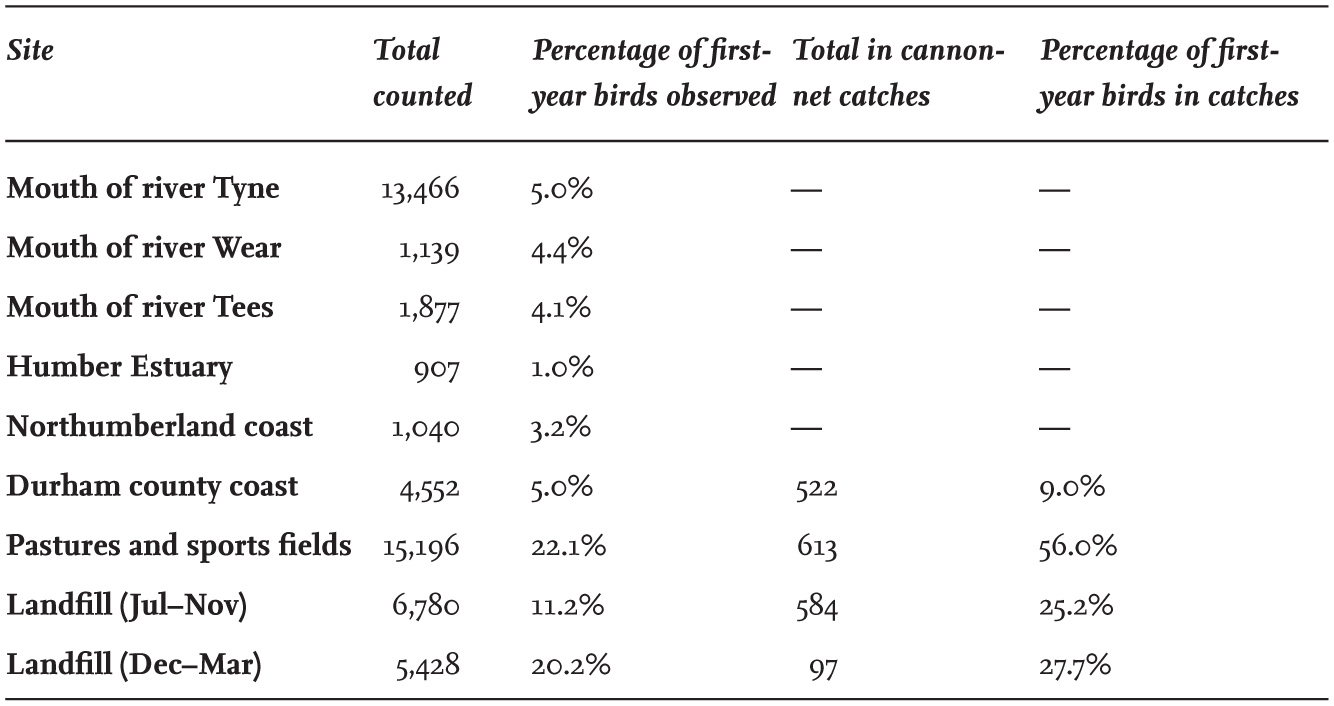
Interestingly, the cannon-net captures of Black-headed Gulls at landfill sites were composed of up to twice the proportion of first-year birds than those counted at nearby loafing sites before making a capture (Table 15). This probably indicates that the young birds take greater advantage of feeding frenzies and possibly have a greater risk of capture because of their less efficient feeding ability, which causes them to stay and feed for longer at landfill sites.
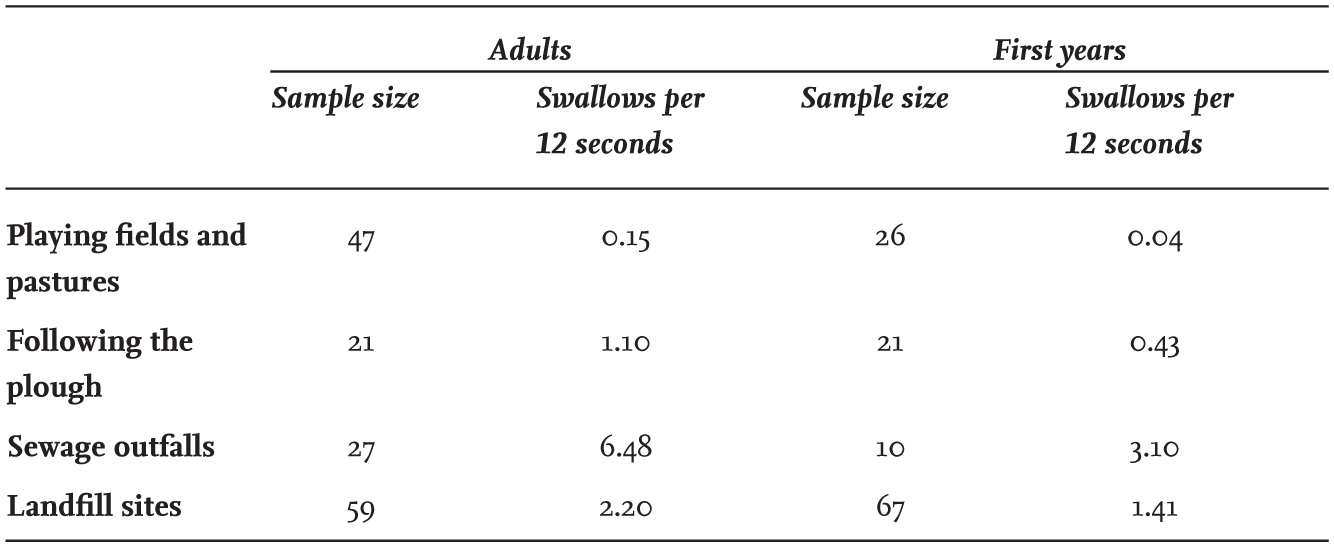
Feeding rates of Black-headed Gulls differ considerably between different sites (Table 16). Items are taken much more rapidly at sewage outfalls, as these sites offer an abundance of readily available food. Following the plough is less successful as it involves a degree of searching or gaining good positions, while walking over short grass on playing fields or pasture involves considerable searching before each food item is discovered. The figures in Table 16 do not take into account the size of each item consumed, although they were likely to be similar on fields and at coastal sites, and smaller at sewage outfalls.
TABLE 16. Feed rates measured as numbers of swallows per 12 seconds by Black-headed Gulls at different feeding sites. First-year birds were slower feeders than adults at all three sites. Data mainly collected by Gabriella MacKinnon and personal unpublished records.
Weight changes
The studies of Black-headed Gulls in north-east England confirmed that adult males are about 17 per cent heavier on average than females throughout the year, and that both sexes are 15–17 per cent heavier in winter than in summer (Fig. 38), reaching a peak weight in December when daylight is shortest. From February onwards, both sexes gradually lose weight, reaching their lowest weight in summer. Recently fledged first-year birds in July are much lighter than adults, but soon catch up, so that by September they have almost reached the weight of adults of the same sex. They then remain marginally lighter throughout the rest of the autumn and winter. The heavier weights in winter are, presumably, caused by an increased layer of subcutaneous fat beneath the skin, which reduces heat loss and acts as a reserve to tide individuals over difficulties encountered in feeding during severe weather.
The low weights of adults in summer does not seem to be a direct response to the stress and effort of breeding, because first-year birds, which do not breed, follow the same trend from November to March. Lower summer weights are achieved by adults by March, before they migrate and have started to visit their colonies. Between March and August, adults maintain the lower weight, and there is only slight evidence that breeding causes further weight loss. One effect of lower body weight in summer is that the energy cost of flying is reduced. This is at a time when food reserves protecting against cold weather and insulation to reduce heat loss are less necessary, and so there is no need for birds to carry the associated extra weight.
In the studies, only minimal differences were apparent in the weights of Black-headed Gulls caught in July and August just after dawn (before they have eaten their first feed of the day) compared to those caught in the afternoon (Table 17). However, as day length becomes shorter and the period when the gulls cannot feed increases, their overnight weight loss increases progressively. By December and January, the overnight weight loss recorded reached 18.3 g, which is an appreciable 6 per cent decrease of the afternoon body weight, at a time when body weight is at a seasonal maximum (Table 17 and Fig. 38).
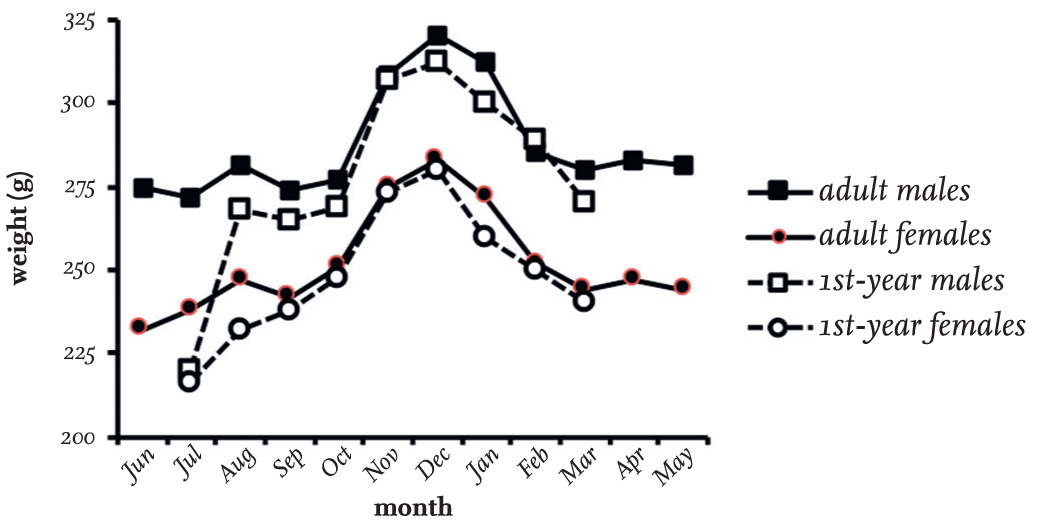
FIG 38. The monthly average weights of adult and first-year male and female Black-headed Gulls in north-east England. Based partly on data analysed by MacKinnon (1986) and on unpublished personal data, totalling 1,953 individuals. Note that few first-year birds were weighed in April, May and June.
There is a widely held view that winter is the period when birds face the highest risk of food shortages, leading to loss of weight and increased mortality. In the case of the Black-headed Gull, however, this is not so. Prolonged periods of hard frost do little to affect the average weight of the birds. Table 18 shows the deviation in weight of Black-headed Gulls from the monthly average in relation to the number of consecutive days with hard ground frosts before capture. Short periods of frosty weather had little impact on their weights, and even with more than four days of continuous hard frosts, their weights had declined by only 3.7 g, or about 1.2 per cent of the body mass expected at that time of year.
TABLE 17. Weight change between dawn and afternoon of adult Black-headed Gulls between July and January. Weights are the average for males and females recorded in north-east England. Significant overnight weight losses are indicated by an asterisk (*).
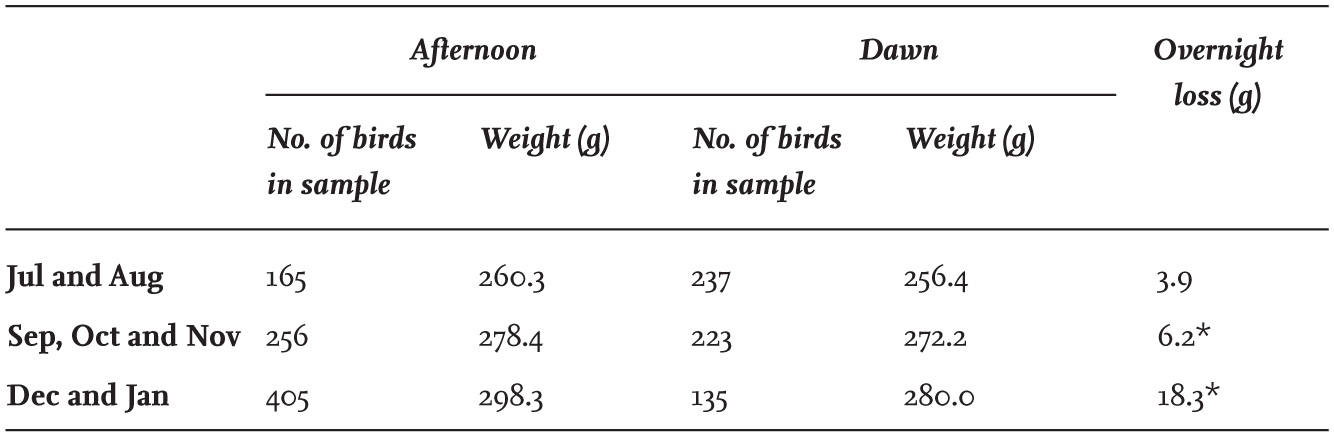
TABLE 18. The effect of consecutive days with hard ground frosts on the weight of Black-headed Gulls. Results are shown as the deviation from the average weight of adults of each sex in the month of capture in north-east England.
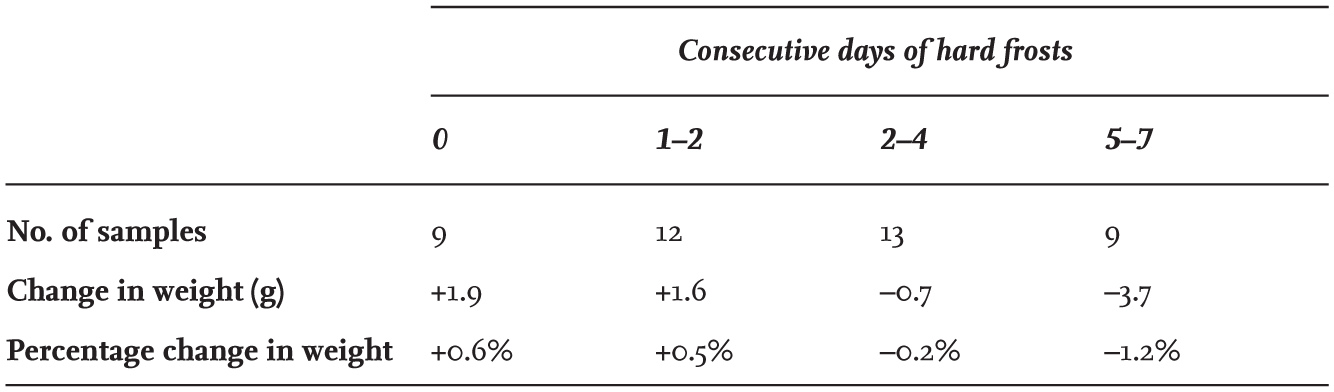
FIG 39. The percentage of the total number of ringed adult Black-headed Gulls reported dead in each month of the year. The distribution suggests that mortality is relatively low during the winter months and then peaks at about twice the winter rate during the breeding season (May to July). Figures are based on 2,575 recoveries of adults and include individuals recovered abroad.
With Black-headed Gulls achieving high body weights in winter and sustaining these during cold weather, this begs the question as to when the risk of mortality is highest in the species. This was first investigated by Jim Flegg and Christopher Cox in 1956–74 (Flegg & Cox, 1975), and the amount of related data have increased since then following the recovery of many more British ringed birds found dead from 1975 to 1984 (Fig. 39). In fact, the seasonal pattern of deaths has changed little since the original analysis, with the numbers reported dying each month during the breeding season (April to July) double those in each month between October and March. In the British climate, Black-headed Gulls are at a greater risk of being killed by predatory mammals while breeding (as found by Hans Kruuk at Ravenglass and Clive Craik in the Hebrides), than by food shortages in winter. While the birds are breeding and are attached to a particular colony site, their feeding areas are restricted. Agricultural fields planted with root or grain crops become unsuitable as feeding areas as the plants grow, while those where grass is allowed to grow tall for hay and silage production also become unsuitable until the first cuts are made. Once the grass is cut, the speed at which the fields are then visited by gulls strongly supports the belief that food may be in short supply during the breeding season.
ANNUAL SURVIVAL RATES
Considering that the Black-headed Gull is so abundant, there is relatively little information on the survival rates for members of the species. The first estimates were published in 1975 by Flegg and Cox, who suggested that the adult survival rate in the south of England was 76 per cent per year. This value was probably low because some recoveries of old birds were still to be reported, and in addition, doubts now exist about the durability of the rings then in use, which may have reduced the estimated survival rate (see below). More recently, Anne-Caroline Prévot-Julliard, Jean-Dominique Lebreton and Roger Pradel (1998) estimated a 90 per cent annual adult survival rate in France.
Gabriella MacKinnon and I used British BTO ringing recoveries to estimate the annual survival rates of Black-headed Gulls ringed as nestlings in the UK. The rate in the bird’s first year of life proved to be low, followed by a constant rate of 84 per cent for the next 10 years of life (MacKinnon & Coulson, 1987). Thereafter, the annual survival rate declines progressively, with the oldest bird reported at 22 years of age; this is well below a maximum age of 33 years reported by Klaas van Dijk and Rob Voesten in 2014. There are two possible explanations for this pattern. One is that senility sets in after Black-headed Gulls reach about 10 years of age. The second is that the rings on the gulls start to be lost or become illegible after 10 years, resulting in fewer reports of old birds that are of this age in the population. We encountered a similar problem with aluminium and Monel rings used on Kittiwakes and Herring Gulls in the past, and examined both aluminium and Monel rings used by BTO ringers recovered during our studies on Black-headed Gulls in north-east England. We concluded that ring wear and loss was a problem and that a few individuals probably outlived the life of the rings used to mark them. As a result, survival rates were probably underestimated. In addition, based on information available for other seabirds, it is considered unlikely that senility occurs in 10-year-old individuals. We also found that Monel rings, which were introduced to overcome the wear found on aluminium rings, actually lost weight at a similar rate to the aluminium ones and were therefore little improvement.



