 Полная версия
Полная версияNature's Teachings
Below the vegetable comes a rather celebrated animal cistern, namely, a portion of one of the stomachs of a Camel.
It exactly corresponds with that part of an ox which butchers call “honey-comb tripe,” and consists of a multitude of cells, which can be closed or opened at will. When the camel takes in its provision of water, it can treat this portion of the stomach much as the hive bee treats the honey-bag, and fill its cells with water.
By degrees, when it finds the necessity for moisture, it can squeeze the water out of these receptacles into the digestive portion of the interior, and so can sustain life for a wonderfully long time under conditions which would kill any other animal. I may remark, by the way, that the amount which a camel can drink, and the length of time through which it can endure its desert life, have been much exaggerated. There is another point to be considered, namely, the curious resemblance between these cells and the honey-comb of the hive bee. Every one knows that honey, no matter how tightly closed, will crystallize and lose its best qualities if kept in jars, whereas if it be allowed to remain in the waxen comb, where it is divided into very small portions, it will remain good for years.
It is just the same with the cells of the camel’s stomach, they being able to preserve water in a pure state by distributing it among a number of small cells, which can be opened or closed at will.
Then we come to the various means of obtaining water.
Reference has already been made to the Filter, by which foul water can be made pure for human consumption, and we will therefore pass to another mode of obtaining pure water, namely, the Still.
In former days, if there were a failure of the supply of fresh water on board ship, the whole of the occupants must necessarily perish. Now, however, no such danger exists, as every well-furnished ship carries at least one Still, by means of which the sea-water can be made to abandon its salt, and to give out nothing but pure water fit for drinking.
Even in cases where no regular Still has been on board, an extemporised Still has been made from a kettle, a gun barrel, or piece of lead piping, or anything of a similar nature.
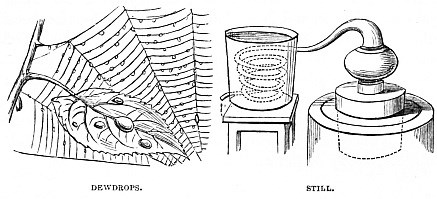
The principle of the Still is simple enough, and is shown by the diagram, rather than drawing, on the right hand of the illustration. There is a vessel in which liquid is boiled. From the upper part of it rises a tube through which the steam must pass as it is generated. The tube in question is generally of considerable length, and is coiled inside a vessel filled with cold water, rendered colder by ice, if possible.
As the steam passes through the cold tube condensation takes place, and it becomes liquid again, but deprived of its heavier particles, so that if sea-water be placed in the still, the salt is left in the vessel, and nothing but pure water passes through the tube. In dissecting-rooms a small still is almost invariably kept. Many preparations are of such a nature that the spirit in which they are placed becomes discoloured, and has to be repeatedly changed. Now, even methylated spirit is an expensive article, and therefore, instead of being thrown away, the discoloured spirit is placed in the still, and reproduced in a clean and transparent state.
Nature affords innumerable examples of distillation, the chief of which are the Dewdrops which have already been mentioned. During the daytime the air is full of moisture drawn by the sunbeams from ocean. We cannot see it, but it is there, and when the chill of night cools the various trees, herbage, and other such objects, the aërial moisture is condensed upon them, which is then known by the name of Dew.
On the left hand of the illustration are shown the tiny Dewdrops as hanging on the slight threads of a spider’s web, and collected in larger drops upon a leaf.
There are many other familiar examples of the principle of condensation, the commonest of which is the so-called steam as it pours from the spout of a kettle. In point of fact, it is not steam at all, but only water condensed into very small drops. At the orifice of the kettle it is quite invisible, but when it passes into the air, and is condensed, the tiny globules become visible. The same fact may be noticed in the Napier’s Coffee Machine, which has already been mentioned. When the water is boiling in the glass globe no steam is visible, though the upper portion of the globe is entirely filled by it. But, no sooner is the cork removed, and the steam allowed to escape, than it at once becomes visible as a white cloud, being, indeed, a miniature copy of the rain-clouds that float above us.
Then there is that mostly invisible passage of liquid through the multitudinous pores of the body, which is generally known as perspiration. It is invisible in warm weather, but on a cold day is as visible as a rain cloud.
The Turkish Bath affords a good example of this fact. Sometimes the hottest room attains a temperature of 250° or more, water boiling at 212°. When a bather goes into that room, he appears to have a perfectly dry skin, the moisture being in the form of invisible steam, and swept off as soon as it is generated.
But, if he passes at once into the cold room, he is so enveloped in vapour that for a few moments he is wrapped in it as in a cloud, and can scarcely be seen, the vapour having been condensed by the cold air.
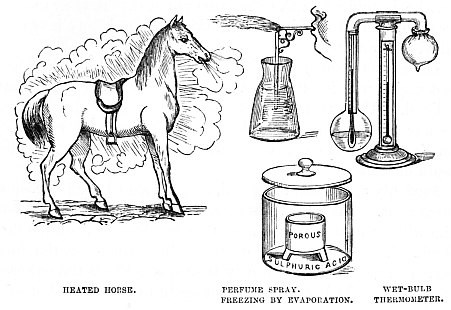
A very familiar instance of this sudden condensation may be seen in the streets of London on any winter day. There may be a couple of omnibus horses, nearly at the end of their day’s work, and quite tired out. Suddenly they are pulled up by the driver, and as suddenly disappear for a moment or two, being concealed in a cloud of moisture proceeding from their bodies. Of course in a hot day there is more of the moisture, but the warmth of the atmosphere prevents it from condensation, and so it is not visible.
One valuable property of the system of evaporation and condensation is its cooling power. Thus it is that a person who is ill with fever tosses about with a burning skin until the pores of the body act, and allow the normal moisture to pass through them. Then the body cools by evaporation, and the patient begins to amend.
So it is that the bather can endure in the Turkish bath a heat so great that a glass of water, if held in the hand, would speedily boil, and a piece of meat be cooked in about the same period. But, if the air were not dry enough to carry off the perspiration, the bather would be scalded to death.
A most valuable adaptation of the principle is shown in the little glass machine for dispersing perfumes in the form of spray. In cases of headache it is almost invaluable, the spray cooling the heated forehead, like magic, and at the same time filling the room with the grateful perfume.
It has even a greater claim to human gratitude, as I can personally testify. I have the strongest objection to a surgeon’s knife, especially when I know, from sad experience, that he is going to make very free use of it. But, on the last occasion, I cared nothing for it, owing to the happy invention called Ether Spray.
The effects were remarkable. First, a delicious cooling of a spot raging with internal fires. Then it was rather colder than I liked. Then it was much colder than I liked. Then it became almost too cold to bear, reminding me of my childhood’s feet on the outside of the Birmingham coach in the depth of winter.
Suddenly all sensation ceased, and the skin became white as parchment. Out came the surgeon’s bistoury, and I looked at him with as calm composure as if he had been whittling a deal plank. There was absolutely no feeling whatever, the local nerves having been temporarily frozen, so great is the power of evaporation. If it ever be my lot again to endure cold steel, I shall have the ether spray.
On the extreme right of the illustration is seen the “Wet-bulb” Thermometer, which carries out the same principle, the thermometer being double, and one bulb being covered with a wet envelope, while the other is dry.
Below is one of the many inventions for making artificial ice, all of them depending on the cooling power of evaporation. Perhaps some of my readers may have seen molten iron poured over the human hand without doing the least harm, or mercury frozen in a red, or rather a white, hot vessel. Both these phenomena are due to the cooling power of evaporation, which is made to act with extreme rapidity, and so absorbs the heat until even mercury is rendered solid, and can be cast in a mould like a leaden bullet.
In the accompanying illustration we have an example of the Condensating principle as applied to the steam-engine, and popularly known as the “Low-pressure Engine.” In this case force is reconverted, so to speak, and, if a cubic inch of water has been converted by heat into a cubic foot of steam, creating a pressure in one direction, it can be reconverted by cold, and so produce a pressure in another direction.
It is owing to this fact that some parts of the world are always hot and always wet, Guiana being a striking example.
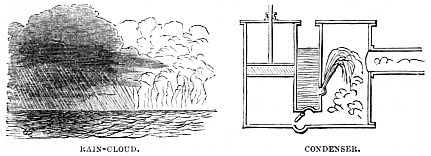
The wind blows over the ocean, absorbing moisture as a sponge does water. As it passes from the sea over the land, it is met by secondary mountain ranges, too low to arrest its progress altogether, and high enough to have their summits clothed in eternal snows. As soon, therefore, as the warm, water-laden winds pass over these mountains, the moisture is condensed by their frozen tips, and down rushes the rain in torrents.
Even in our own temperate land we can often trace the cause of a heavy rain to the presence of a lofty hill, or even an exceptionally tall spire. The moist climate of Oxford has been attributed by scientific men quite as much to its spires and towers as to its low-lying situation.
Now we come to the various modes of extracting the water which is laid up within the earth, and which only slowly ascends to the surface when drawn up by the heat of the sun.
Water is everywhere, but the depths at which it is found are vastly different. For example, at one house in which I lived it was not possible to dig for three feet without coming to water. In another, no water was found within some two hundred feet, and, as I several times relieved the old gardener of the task of drawing the water for the day’s consumption, I have reason to remember the depth.
The pail, rope, and winch which were in use at that time—and may be still, to the sorrow of the gardener—are but a sort of semi-savage way of procuring water from the depths of the earth. It is a well-known fact that under certain conditions water always finds its own level, minus the friction of the channel through which it passes. On this principle all fountains are made. Those, for example, at the Crystal Palace, which fling their waters to such a height, are fed from tanks on the summit of the two great water towers. And, were it not for the friction of the water in the tubes, and that of the air, the fountains would rise as high as the tanks from which they are fed.
Such is the case with springs, especially with those of an intermittent character, in which latter instance the rushing of the water is exactly coincident with the filling of the hidden tank which supplies it.
The modern Hydrant system, which bids fair to supersede the cumbrous machinery of fire-engines, even when worked by steam, is based on the same principle. The water-tanks are placed at such a height that, when a hose is attached, and the tap turned, the water can be thrown over the roof of the highest building. Such hydrants have been attached to Canterbury Cathedral since the fire which so nearly consumed that magnificent and venerable building.
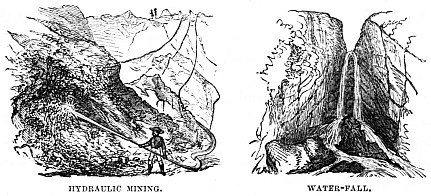
A very remarkable use has been made of this power of water in mining operations. Most of my readers know that in gold mines the metal is chiefly found scattered among quartz, one of the hardest of the minerals. The usual plan has been to dig out the quartz, pound it to powder with specially devised machines called “stamps,” to pass the powder through mercury, which amalgamated with the gold, and gave it up again on being heated to a certain temperature.
Now a different mode of mining is brought into operation, the pickaxe, spade, and stamps, with all their expensive machinery, being abandoned, and water made to do the duty of all three, some ingenious individual having noticed the effect which water has on the hardest rock.
Such, for example, is the case with those wonderful Victoria Falls of Africa, where the rushing water has cut its sinuous channel through so many hundreds of yards of rock. Such, also, is the case with the more celebrated, but not so wonderful, Falls of Niagara, which have been gradually working their way backwards, having worn away the rocks over which they fall, and which are shown to be many miles away from the spot where the river first discharged itself over the cliff.
In fact, it is well known that the Falls are receding at a definite rate annually, and that the rate has been calculated with scientific accuracy. The cliffs of our own coasts-say of Margate or Ramsgate—crumble away with equally calculable speed.
In the hydraulic mining system large tanks are erected, at least two hundred feet above the level of the mine. From these tanks proceed pipes, terminated by hose, just like those of our ordinary fire-engines. The miners, instead of using pickaxe or crowbar, simply direct the streams of water against the solid rock. Their effect is tremendous. They tear it to powder, and carry it down the wooden troughs called “flumes,” in which the mercury is so arranged that not a single atom of quartz rock can pass without having its gold extracted.
The following graphic account of Hydraulic Mining at Nevada is taken from Mr. J. K. Lord’s “Naturalist in British Columbia:”—
“Near Nevada are the famed Hydraulic washings. The gold is disseminated through terraces of shingle conglomerates, often three hundred feet in thickness. These terraces are actually washed entirely off the face of the country by propelling jets of water against them, forced by pressure through a nozzle.
“To accomplish this, the water is brought in canals, tunnels, and wooden aqueducts, often forty miles away from the ‘draft.’ This supply of water the miners rent.
“As we near the washing spot, in every direction immense hose, made of galvanized iron, and canvas tubes six feet round, coil in all directions over the ground like gigantic serpents, converging towards a gap, where they disappear.
“On reaching this gap, I look down into a basin or dry lake, three hundred feet below me. The hose hangs down this cliff of shingle, and following its course by a zigzag path, I reach a plateau of rock, from which the shingle has already been washed.
“A man stands at the end of each hose, that has for its head a brass nozzle. With the force of cannon-shot, water issues in a large jet from this tube, and propelled against the shingle, guided by the men, washes it away as easily as we could sweep a molehill from off the grass.
“The stream of water, bearing with it the materials washed from out the cliff, runs through wooden troughs called ‘flumes,’ floored with granite. These ‘flumes’ extend six miles. Men are stationed at regular distances to fork out the heavy stones.
“Throughout its entire length, transverse strips of wood dam back a tiny pond of mercury. These are called ruffles—gold-traps, in other words, that seize on the fine dust-gold distributed through the shingle. The flumes are cleaned about once a month, and the gold extracted from the mercury.
“I try with a powerful lens to detect gold amidst the material they are washing, but not a trace is discoverable, and yet it pays an immense profit to the gold-washers.”
There are two more modes of extracting water, which will be but cursorily mentioned.
The reader will remember that water finds its own level, and that the terrific power of hydraulic mining is owing to the fact that the water expends its force against the solid rock instead of ascending into the air.
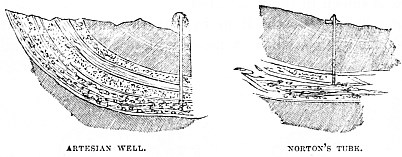
It is now found that, even without artificial assistance, water has a habit of finding its own level, and that, if it be allowed its own course, it will contrive to find its way nearly to the highest point whence it derived its origin. On this principle are based the Artesian Wells, which, when they “strike water,” spurt it up in a torrent, as is the case with the now celebrated Norton Tubes, which are screwed down into the earth like hollow gimlets, and which always contrive to extract the water hidden beneath the surface of the earth.
The success of our army in Abyssinia was greatly owing to these Norton Tubes, which, being of small diameter and of peculiar make, could be screwed into the ground when the troops made a halt, unscrewed when they left the spot, and used again for the next halt.
Similarly, the French used the Artesian-well system with wonderful success in Northern Africa. Water is the chief necessity of life in that part of the world, and a nation who could cause pure cold water to spring out of the hot and thirsty sands was naturally looked upon as something more than human.
Yet the principle was exactly the same in both cases. Water is always latent somewhere beneath the surface of the earth, and, if a tube can be driven deep enough, the water will come up it.
The accompanying illustration shows the Artesian Well and Norton’s Tube, and their similitude in principle, the tube penetrating through various layers of soil, until it reaches the water which it seeks.
Then there is another way by which water can be made to force itself to a considerable height. Not being much of a mathematician, I do not recollect the exact proportional height to which a stream of water may raise itself, but if any one can secure a fall of some eight or ten feet, he can furnish his house with water by means of the “Ram,” a chart of which is shown in the illustration.
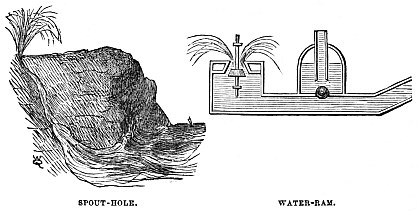
The principle of the Ram is, that the water is allowed to flow down a tube, when it meets with a valve. This valve is suddenly closed by the pressure, and the water is forced onwards by the shock. Much water escapes at each blow of the valve, but that does not signify.
The force of water thus suddenly stopped is hardly appreciated. Even in ordinary houses the sudden turning of a water-tap has been known to burst the pipe and deluge the house with water.
In Nature a similar effect is produced, called popularly the “Spout-hole.”
It is a hole or tunnel on the seashore, passing upwards from the level of the sea to the summit of the cliff.
When the waves are urged against the tunnel by the wind, the water is dashed into it. Being partially checked by the friction, which acts exactly like the water that is checked by the Ram, the wave hurls itself up the channel, and flies out in showers of spray, high above the level of the original wave which caused it.
In the illustration are shown the Water-ram with its globular valve, and the safety or escape valve of the waste water. On the left is shown one of the natural Spout-holes, with the water dashing through its tunnel into a mass of spray.
CHAPTER XI.
AËROSTATICS.—WEIGHT OF AIR.—EXPANSION BY HEAT
Ascent and Descent.—The Balloon and the Parachute.—Description of the Balloon.—The Montgolfier Balloon.—Causes of its Abandonment.—The Gas Balloon.—Hydrogen Gas and its Manufacture.—The Gossamer Spider.—Reasons of its Ascent and Descent.—Many Species of Gossamers.—Description of the Parachute.—Its Mode of Action.—A Balloon converted into a Parachute.—Toy Parachutes.—Natural Parachutes.—The Dandelion Seed and its Structure.—The Flying Squirrel.—The Flying Monkey.—Flying Mice and Flying Opossums.—The Flying Dragon and its Pseudo-wings.—The Flying Frog.—Weight of Air.—Pressure per Square Inch.—The Air Ocean and its Storms.—Principle of Air-currents.—The Sun, the Earth, and the Air.—Ventilation of Mines.—Choke-damp and Fire-damp.—The Air-shafts.—Chimneys of Factories.—The Steam-blast.—The Barometer, and Mode of its Construction.—Water and Mercury.—Sucking Eggs and Sugar-cane.—Expansion of Water and Metals by Heat.—The Thermometer.—Wheel-making.
AërostaticsWE will begin this chapter with the only two modes at present known by which man can ascend from the earth or descend to it with safety, namely, the Balloon and the Parachute, the latter being generally attached to the former, and detachable at pleasure.
The Balloon is, in fact, as its name imports, a large, hollow, air-tight ball, filled with some substance lighter than ordinary air. The original Balloons by Montgolfier were filled with heated air exactly like our toy fire-balloons. Just as the supply of hot air is kept up in them by a sponge dipped in lighted spirits of wine, so in Montgolfier’s balloons the same object was attained by straw which was kept continually burning in a grate.
There were, however, two disadvantages about this plan. The first was the great danger of fire, which on one occasion did ignite a balloon when at a great height. The second was the perpetual labour required in keeping the fire alight. Straw burns very rapidly, and so the aëronaut had no opportunity of making those meteorologic observations in which consist almost the entire value of the balloon.
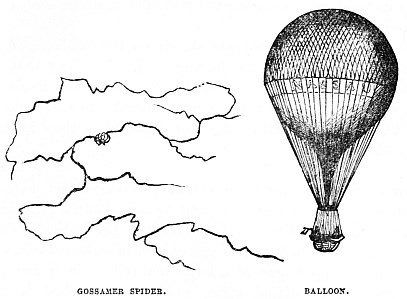
Then it was thought that hydrogen gas, being about fourteen times lighter than ordinary air, would answer the purpose, and such has proved to be the case. Formerly the gas was made at great expense from sulphuric acid and zinc, but it is now found that the common coal-gas is quite as efficient, very much cheaper, and fills the balloon much more rapidly.
The same principle, though not the same form, is found in Nature.
There are certain tiny spiders called Gossamers, which have a curious power of floating in the air. They have been seen on the tops of lofty spires, and they are sometimes so numerous that the air is full of their floating webs, and the ground is white with those that have descended.
Their mode of ascent is this. They climb to the top of some elevated object, if it be only a grass-blade. They then pour out a tuft of long, slender threads, which shortly begin to tend upwards. As soon as the Spider feels the pull, it crawls upon the web, and sails away into the air. The duration and height of the ascent depend much on the wind and character of the atmosphere.
The web ascends because it is for the time lighter than the atmosphere. But, as it gradually becomes laden with the moisture that more or less fills the air, it becomes heavier than the atmosphere, and gently sinks to the ground.
What may be the object of these aërial voyages no one knows. They may be for the purpose of capturing minute insects, or they may be for mere amusement. But in either case they are highly instructive, as showing the principle on which the balloon was framed.
The little Gossamer Spider is shown on the left hand of the illustration, clinging to its floating web. I believe that the Gossamer is not a single species of Spider, but that there are many species which deserve the name, being able to float in the air when they are small, but losing that capacity as they increase in size and weight.
Now we come to another branch of the same subject, namely, the safe descent from a great height by means of the Parachute.
On the right hand of the illustration is the ordinary Parachute as it appears when open and closed, in either case having somewhat the appearance of a large umbrella. It is hung to the balloon in its closed state, and when detached it falls rapidly for a yard or two with startling rapidity. The pressure of the air thus forces the ribs open, and gives sufficient assistance to the atmosphere to insure a gentle fall.

