
Полная версия:
The Tao of Physics
In the orbits, the electron waves have to be arranged in such a way that ‘their ends meet’, i.e. that they form patterns known as ‘standing waves’. These patterns appear whenever waves are confined to a finite region, like the waves in a vibrating guitar string, or in the air inside a flute (see diagram opposite). It is well known from these examples that standing waves can assume only a limited number of well-defined shapes. In the case of the electron waves inside an atom, this means that they can exist only in certain atomic orbits with definite diameters. The electron of a hydrogen atom, for example, can only exist in a certain first, second or third orbit, etc., and nowhere in between. Under normal conditions, it will always be in its lowest orbit, called the ‘ground state’ of the atom. From there, the electron can jump to higher orbits if it receives the necessary amount of energy, and then the atom is said to be in an ‘excited state’ from which it will go back to its ground state after a while, the electron giving off the surplus energy in the form of a quantum of electromagnetic radiation, or photon. The states of an atom, i.e. the shapes and mutual distances of its electron orbits, are exactly the same for all atoms with the same number of electrons. This is why any two oxygen atoms, for example, will be completely identical. They may be in different excited states, perhaps due to collisions with other atoms in the air, but after a while they will invariably return to exactly the same ground state. The wave nature of the electrons accounts thus for the identity of atoms and for their great mechanical stability.
A further characteristic feature of atomic states is the fact that they can be completely specified by a set of integral numbers, called ‘quantum numbers’, which indicate the location and shape of the electron orbits. The first quantum number is the number of the orbit and determines the energy an electron must have to be in that orbit; two more numbers specify the detailed shape of the electron wave in the orbit and are related to the speed and orientation of the electron’s rotation.* The fact that these details are expressed by integral numbers means that the electron cannot change its rotation continuously, but can only jump from one value to another, just as it can only jump from one orbit to another. Again the higher values represent excited states of the atom, the ground state being the one where all the electrons are in the lowest possible orbits and have the smallest possible amounts of rotation.
Tendencies to exist, particles reacting to confinement with motion, atoms switching suddenly from one ‘quantum state’ to another, and an essential interconnectedness of all phenomena—these are some of the unusual features of the atomic world. The basic force, on the other hand, which gives rise to all atomic phenomena is familiar and can be experienced in the macroscopic world. It is the force of electric attraction between the positively charged atomic nucleus and the negatively charged electrons. The interplay of this force with the electron waves gives rise to the tremendous variety of structures and phenomena in our environment. It is responsible for all chemical reactions, and for the formation of molecules, that is, of aggregates of several atoms bound to each other by mutual attraction. The interaction between electrons and atomic nuclei is thus the basis of all solids, liquids and gases, and also of all living organisms and of the biological processes associated with them.
In this immensely rich world of atomic phenomena, the nuclei play the role of extremely small, stable centres which constitute the source of the electric force and form the skeletons of the great variety of molecular structures. To understand these structures, and most of the natural phenomena around us, it is not necessary to know more about the nuclei than their charge and their mass. In order to understand the nature of matter, however, to know what matter is ultimately made of, one has to study the atomic nuclei which contain practically all of its mass. In the 1930s, after quantum theory had unravelled the world of atoms, it was therefore the main task of physicists to understand the structure of nuclei, their constituents and the forces which hold them together so tightly.
The first important step towards an understanding of nuclear structure was the discovery of the neutron as the second constituent of the nucleus, a particle which has roughly the same mass as the proton (the first nuclear constituent)—about two thousand times the mass of the electron—but does not carry an electric charge. This discovery not only explained how the nuclei of all chemical elements were built up from protons and neutrons, but also revealed that the nuclear force, which kept these particles so tightly bound within the nucleus, was a completely new phenomenon. It could not be of electromagnetic origin since the neutrons were electrically neutral. Physicists soon realized that they were here confronted with a new force of nature which does not manifest itself anywhere outside the nucleus.
An atomic nucleus is about one hundred thousand times smaller than the whole atom and yet it contains almost all of the atom’s mass. This means that matter inside the nucleus must be extremely dense compared to the forms of matter we are used to. Indeed, if the whole human body were compressed to nuclear density it would not take up more space than a pinhead. This high density, however, is not the only unusual property of nuclear matter. Being of the same quantum nature as electrons, the ‘nucleons’—as the protons and neutrons are often called—respond to their confinement with high velocities, and since they are squeezed into a much smaller volume their reaction is all the more violent. They race about in the nucleus with velocities of about 40,000 miles per second! Nuclear matter is thus a form of matter entirely different from anything we experience ‘up here’ in our macroscopic environment. We can, perhaps, picture it best as tiny drops of an extremely dense liquid which is boiling and bubbling most fiercely.
The essential new aspect of nuclear matter which accounts for all its unusual properties is the strong nuclear force, and the feature that makes this force so unique is its extremely short range. It acts only when the nucleons come very near to each other, that is, when their distance is about two to three times their diameter. At such a distance, the nuclear force is strongly attractive, but when the distance becomes less the force becomes strongly repulsive so that the nucleons cannot approach each other any closer. In this way, the nuclear force keeps the nucleus in an extremely stable, though extremely dynamic equilibrium.
The picture of matter which emerges from the study of atoms and nuclei shows that most of it is concentrated in tiny drops separated by huge distances. In the vast space between the massive and fiercely boiling nuclear drops move the electrons. These constitute only a tiny fraction of the total mass, but give matter its solid aspect and provide the links necessary to build up the molecular structures. They are also involved in the chemical reactions and are responsible for the chemical properties of matter. Nuclear reactions, on the other hand, generally do not occur naturally in this form of matter because the available energies are not high enough to disturb the nuclear equilibrium.
This form of matter, however, with its multitude of shapes and textures and its complicated molecular architecture, can exist only under very special conditions, when the temperature is not too high, so that the molecules do not jiggle too much. When the thermal energy increases about a hundredfold, as it does in most stars, all atomic and molecular structures are destroyed. Most of the matter in the universe exists, in fact, in a state which is very different from the one just described. In the centre of the stars exist large accumulations of nuclear matter, and nuclear processes which occur only very rarely on earth predominate there. They are essential for the great variety of stellar phenomena observed in astronomy, most of which arise from a combination of nuclear and gravitational effects. For our planet, the nuclear processes in the centre of the Sun are of particular importance because they furnish the energy which sustains our terrestrial environment. It has been one of the great triumphs of modern physics to discover that the constant energy flow from the Sun, our vital link with the world of the very large, is a result of nuclear reactions, of phenomena in the world of the infinitely small.
In the history of penetrating into this submicroscopic world, a stage was reached in the early 1930s when scientists thought they had now finally discovered the ‘basic building blocks’ of matter. It was known that all matter consisted of atoms and that all atoms consisted of protons, neutrons and electrons. These so-called ‘elementary particles’ were seen as the ultimate indestructible units of matter: atoms in the Demo-critean sense. Although quantum theory implies, as mentioned previously, that we cannot decompose the world into independently existing smallest units, this was not generally perceived at that time. The classical habits of thought were still so persistent that most physicists tried to understand matter in terms of its ‘basic building blocks’, and this trend of thought is, in fact, quite strong even today.
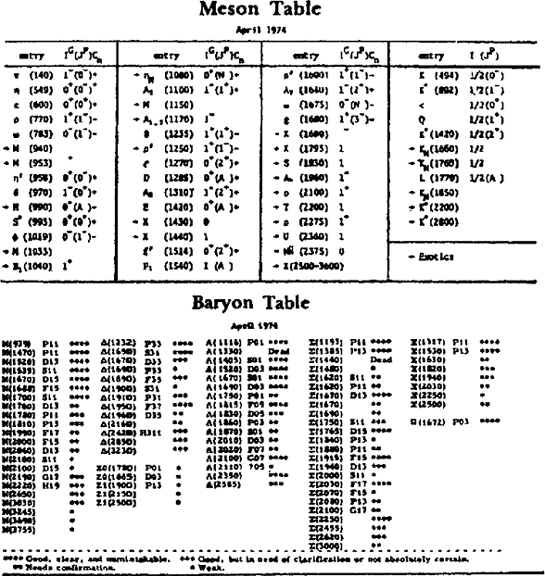
Two further developments in modern physics have shown, however, that the notion of elementary particles as the primary units of matter has to be abandoned. One of these developments was experimental, the other theoretical, and both began in the 1930s. On the experimental side, new particles were discovered as physicists refined their experimental techniques and developed ingenious new devices for particle detection. Thus the number of particles increased from three to six by 1935, then to eighteen by 1955, and today we know over two hundred elementary’ particles. The two tables opposite, taken from a recent publication,11 show most of the particles known today. They illustrate convincingly that the adjective ‘elementary’ is no longer very attractive in such a situation. As more and more particles were discovered over the years, it became clear that not all of them could be called ‘elementary’, and today there is a widespread belief among physicists that none of them deserves this name.
This belief is enforced by the theoretical developments which paralleled the discovery of an ever-increasing number of particles. Soon after the formulation of quantum theory, it became clear that a complete theory of nuclear phenomena must not only be a quantum theory, but must also incorporate relativity theory. This is because the particles confined to dimensions of the size of nuclei often move so fast that their speed comes close to the speed of light. This fact is crucial for the description of their behaviour, because every description of natural phenomena involving velocities close to the speed of light has to take relativity theory into account. It has to be, as we say, a ‘relativistic’ description. What we need, therefore, for a full understanding of the nuclear world is a theory which incorporates both quantum theory and relativity theory. Such a theory has not yet been found, and therefore we have as yet been unable to formulate a complete theory of the nucleus. Although we know quite a lot about nuclear structure and about the interactions between nuclear particles, we do not yet understand the nature and complicated form of the nuclear force on a fundamental level. There is no complete theory of the nuclear particle world comparable to quantum theory for the atomic world. We do have several ‘quantum-relativistic’ models which describe some aspects of the world of particles very well, but the fusion of quantum and relativity theory into a complete theory of the particle world is still the central problem and great challenge of modern fundamental physics.
Relativity theory has had a profound influence on our picture of matter by forcing us to modify our concept of a particle in an essential way. In classical physics, the mass of an object had always been associated with an indestructible material substance, with some ‘stuff’ of which all things were thought to be made. Relativity theory showed that mass has nothing to do with any substance, but is a form of energy. Energy, however, is a dynamic quantity associated with activity, or with processes. The fact that the mass of a particle is equivalent to a certain amount of energy means that the particle can no longer be seen as a static object, but has to be conceived as a dynamic pattern, a process involving the energy which manifests itself as the particle’s mass.
This new view of particles was initiated by Dirac when he formulated a relativistic equation describing the behaviour of electrons. Dirac’s theory was not only extremely successful in accounting for the fine details of atomic structure, but also revealed a fundamental symmetry between matter and antimatter. It predicted the existence of an anti-electron with the same mass as the electron but with an opposite charge. This positively charged particle, now called the positron was indeed discovered two years after Dirac had predicted it. The symmetry between matter and antimatter implies that for every particle there exists an antiparticle with equal mass and opposite charge. Pairs of particles and antiparticles can be created if enough energy is available and can be made to turn into pure energy in the reverse process of annihilation. These processes of particle creation and annihilation had been predicted from Dirac’s theory before they were actually discovered in nature, and since then they have been observed millions of times.
The creation of material particles from pure energy is certainly the most spectacular effect of relativity theory, and it can only be understood in terms of the view of particles outlined above. Before relativistic particle physics, the constituents of matter had always been considered as being either elementary units which were indestructible and unchangeable, or as composite objects which could be broken up into their constituent parts; and the basic question was whether one could divide matter again and again, or whether one would finally arrive at some smallest indivisible units. After Dirac’s discovery, the whole question of the division of matter appeared in a new light. When two particles collide with high energies, they generally break into pieces, but these pieces are not smaller than the original particles. They are again particles of the same kind and are created out of the energy of motion (‘kinetic energy’) involved in the collision process. The whole problem of dividing matter is thus resolved in an unexpected sense. The only way to divide subatomic particles further is to bang them together in collision processes involving high energies. This way, we can divide matter again and again, but we never obtain smaller pieces because we just create particles out of the energy involved in the process. The subatomic particles are thus destructible and indestructible at the same time.
This state of affairs is bound to remain paradoxical as long as we adopt the static view of composite ‘objects’ consisting of basic building blocks’. Only when the dynamic, relativistic view is adopted does the paradox disappear. The particles are then seen as dynamic patterns, or processes, which involve a certain amount of energy appearing to us as their mass. In a collision process, the energy of the two colliding particles is redistributed to form a new pattern, and if it has been increased by a sufficient amount of kinetic energy, this new pattern may involve additional particles.
High-energy collisions of subatomic particles are the principal method used by physicists to study the properties of these particles, and particle physics is therefore also called ‘high-energy physics’. The kinetic energies required for the collision experiments are achieved by means of huge particle accelerators, enormous circular machines with circumferences of several miles in which protons are accelerated to velocities near the speed of light and are then made to collide with other protons or with neutrons. It is impressive that machines of that size are needed to study the world of the infinitely small. They are the supermicroscopes of our time.
Most of the particles created in these collisions live for only an extremely short time—much less than a millionth of a second—after which they disintegrate again into protons, neutrons and electrons. In spite of their exceedingly short lifetime, these particles can not only be detected and their properties measured but are actually made to leave tracks which can be photographed! These particle tracks are produced in so-called bubble chambers in a manner similar to the way a jet plane makes a trail in the sky. The actual particles are many orders of magnitude smaller than the bubbles making up the tracks, but from the thickness and curvature of a track physicists can identify the particle that caused it. The picture opposite shows such bubble chamber tracks. The points from which several tracks emanate are points of particle collisions, and the curves are caused by magnetic fields which the experimenters use to identify the particles. The collisions of particles are our main experimental method to study their properties and interactions, and the beautiful lines, spirals and curves traced by the particles in bubble chambers are thus of paramount importance for modern physics.
The high-energy scattering experiments of the past decades have shown us the dynamic and ever-changing nature of the particle world in the most striking way. Matter has appeared in these experiments as completely mutable. All particles can be transmuted into other particles; they can be created from energy and can vanish into energy. In this world, classical concepts like ‘elementary particle’, ‘material substance’ or ‘isolated object’, have lost their meaning; the whole universe appears as a dynamic web of inseparable energy patterns. So far, we have not yet found a complete theory to describe this world of subatomic particles, but we do have several theoretical models which describe certain aspects of it very well. None of these models is free from mathematical difficulties, and they all contradict each other in certain ways, but all of them reflect the basic unity and the intrinsically dynamic character of matter. They show that the properties of a particle can only be understood in terms of its activity—of its interaction with the surrounding environment—and that the particle, therefore, cannot be seen as an isolated entity, but has to be understood as an integrated part of the whole.
Relativity theory has not only affected our conception of particles in a drastic way, but also our picture of the forces between these particles. In a relativistic description of particle interactions, the forces between the particles—that is their mutual attraction or repulsion—are pictured as the exchange of other particles. This concept is very difficult to visualize. It is a consequence of the four dimensional space-time character of the subatomic world and neither our intuition nor our language can deal with this image very well. Yet it is crucial for an understanding of subatomic phenomena. It links the forces between constituents of matter to the properties of other constituents of matter, and thus unifies the two concepts, force and matter, which had seemed to be so fundamentally different ever since the Greek atomists. Both force and matter are now seen to have their common origin in the dynamic patterns which we call particles.
The fact that particles interact through forces which manifest themselves as the exchange of other particles is yet another reason why the subatomic world cannot be decomposed into constituent parts. From the macroscopic level down to the nuclear level, the forces which hold things together are relatively weak and it is a good approximation to say that things consist of constituent parts. Thus a grain of salt can be said to consist of salt molecules, the salt molecules of two kinds of atoms, those atoms to consist of nuclei and electrons, and the nuclei of protons and neutrons. At the particle level, however, it is no longer possible to see things that way.
In recent years, there has been an increasing amount of evidence that the protons and neutrons, too, are composite objects; but the forces holding them together are so strong or—what amounts to the same—the velocities acquired by the components are so high, that the relativistic picture has to be applied, where the forces are also particles. Thus the distinction between the constituent particles and the particles making up the binding forces becomes blurred and the approximation of an object consisting of constituent parts breaks down. The particle world cannot be decomposed into elementary components.
In modern physics, the universe is thus experienced as a dynamic, inseparable whole which always includes the observer in an essential way. In this experience, the traditional concepts of space and time, of isolated objects, and of cause and effect, lose their meaning. Such an experience, however, is very similar to that of the Eastern mystics. The similarity becomes apparent in quantum and relativity theory, and becomes even stronger in the ‘quantum-relativistic’ models of subatomic physics where both these theories combine to produce the most striking parallels to Eastern mysticism.
Before spelling out these parallels in detail, I shall give a brief account of the schools of Eastern philosophy which are relevant to the comparison for the reader who is not familiar with them. They are the various schools in the religious philosophies of Hinduism, Buddhism and Taoism. In the following five chapters, the historical background, characteristic features and philosophical concepts of these spiritual traditions will be described, the emphasis being on those aspects and concepts which will be important for the subsequent comparison with physics.
* The reader who finds this preliminary presentation of modern physics too compressed and difficult to understand should not be unduly worried. All of the concepts mentioned in this chapter will be discussed in greater detail later on.
* The hydrogen atom consists of just one proton and one electron.
* The ‘rotation’ of an electron in its orbit must not be understood in the classical sense; it is determined by the shape of the electron wave in terms of the probabilities for the particle’s existence in certain parts of the orbit.
Chapter 5 Hinduism
For an understanding of any of the philosophies to be described, it is important to realize that they are religious in essence. Their main aim is the direct mystical experience of reality, and since this experience is religious by nature, they are inseparable from religion. More than for any other Eastern tradition this is true for Hinduism, where the connection between philosophy and religion is particularly strong. It has been said that almost all thought in India is in a sense religious thought and Hinduism has not only influenced, throughout many centuries, India’s intellectual life, but almost completely determined her social and cultural life as well.
Hinduism cannot be called a philosophy, nor is it a well defined religion. It is, rather, a large and complex socio-religious organism consisting of innumerable sects, cults and philosophical systems and involving various rituals, ceremonies and spiritual disciplines, as well as the worship of countless gods and goddesses. The many facets of this complex and yet persistent and powerful spiritual tradition mirror the geographical, racial, linguistic and cultural complexities of India’s vast subcontinent. The manifestations of Hinduism range from highly intellectual philosophies involving conceptions of fabulous range and depth to the naïve and childlike ritual practices of the masses. If the majority of the Hindus are simple villagers who keep the popular religion alive in their daily worship, Hinduism has, on the other hand, brought forth a large number of outstanding spiritual teachers to transmit its profound insights.

