
Полная версия:
E for Additives

E FOR ADDITIVES
THE BEST-SELLING, AWARD WINNING DEFINITIVE E NUMBER GUIDE
Maurice Hanssen
with Jill Marsden
B.Sc., Dip.Ecol., Dip.Ed.

Copyright
Thorsons Element
An Imprint of HarperCollinsPublishers 1 London Bridge Street London SE1 9GF
The website address is: www.thorsonselement.com
First published October 1984
Twentieth Impression December 1986
Second edition (completely revised and substantially expanded) September 1988
© Maurice Hansen 1987
Maurice Hansen asserts the moral right to be identified as the author of this work
A catalogue record of this book is available from the British Library
All rights reserved under International and Pan-American Copyright Conventions. By payment of the required fees, you have been granted the nonexclusive, nontransferable right to access and read the text of this e-book on-screen. No part of this text may be reproduced, transmitted, downloaded, decompiled, reverse engineered, or stored in or introduced into any information storage and retrieval system, in any form or by any means, whether electronic or mechanical, now known or hereinafter invented, without the express written permission of HarperCollins e-books.

Source ISBN: 9780722515624
Ebook Edition © FEBRUARY 2016 ISBN 9780007381562
Version: 2016-01-05
HarperCollinsPublishers has made every reasonable effort to ensure that any picture content and written content in this ebook has been included or removed in accordance with the contractual and technological constraints in operation at the time of publication.
Dedication
This new edition is dedicated to:
All those consumers and manufacturers who use the E code wisely to improve the quality of food and of health;
the many scientists, manufacturers and consumers who have provided invaluable technical information;
all those journalists and broadcasters who helped begin a food buying revolution with the first edition;
my patient and dedicated publishers;
my Research Assistant, Jill Marsden, B.Sc, who continues to triumph over the problems of organizing ever increasing quantities of often contradictory scientific information;
the distinguished and still crusading former Minister of Health, David Ennals, for his perceptive and kind foreword;
Leslie Kenton for her foreword to the first edition;
Elizabeth Brown and Angela Beazley for their word-processing skills, often under great pressure;
my family for their support.
Contents
Cover
Title Page
Copyright
Dedication
Foreword by Lord Enrols
Foreword to First Edition by Leslie Kenton
Introduction
1. How to Read the Label
2. Why Are There Still Secret Ingredients?
3. Golden Eggs and Pink-Fleshed Fish
4. The Colour Problem
5. Flavourings
6. P for Pesticides
7. Is It Kosher?
8. Do Additives Affect Ability?
9. Hyperactivity in Children
10. The Avoidable 57 Additives
11. The Natural Opportunity for Profit
12. The E Number Categories
Appendix I An Introduction to the Safety Assessment (Toxicity) of Additives by R. D. Combes, Ph.D. (Lond.), M.I.Biol.
Appendix II Warning: Dangerous Food Additives—The Villejuif List
Appendix III The Regulation of Food Additives
References
Bibliography
Useful Addresses
Index
About the Publisher
Foreword
There has been a revolution in the approach to what we eat. A series of reports have clearly established the link between food intake and health. It has been supported by doctors, pharmacists, dietitians and politicians. In 1985 a decision was made to establish an all-party Parliamentary Food and Health Forum, of which I am Chairman, and this has become one of the most active Parliamentary groups. Across the country there is concern at the fat and sugar content of food and a growing awareness that 30 per cent of adults in Britain are overweight. There is also a growing interest in colourings and artificial flavourings—in fact, in every type of additive. With Britain’s appalling record of avoidable diseases, there is now a major campaign linking diet and disease.
This growth in interest has led to a public demand for more information, and since 1962 the EEC has been issuing Directives on additives. Since the beginning of January 1986, most foods have carried a full list of additives, apart from flavourings, described by their E numbers on the package. A great step forward—providing you can fully understand the implication of the E number! For instance, I have aspirin sensitivity, and my wife is asthmatic. So we need to know, for both those conditions tend to bring in their wake sensitivities to certain common food preservatives and colours. The book describes these relationships fully, and in addition makes a convincing case for the full disclosure of ingredients and additives on products where they are not yet required to appear by law, such as in many types of confectionery, alcoholic drinks and medicines.
In 1984, following a great deal of research, Maurice Hanssen’s first edition of E for Additives was published. It was a tremendous success and was a bestseller for many months, along with Frederick Forsyth and Jeffrey Archer. It is still in great demand. It contains just enough essential information about the contents and effects (including adverse effects) of each product to enable the shopper to know just what they are being asked to buy.
But since 1984, research has provided a mass of additional information about E-numbered additives and about the wider implications of the need for certain additives in foods where many manufacturers are able to produce excellent foods without their use. Key issues such as the nutritional consequences of the overuse of additives are explored for the first time in this new edition of E for Additives.
To me, the great merit of Maurice Hanssen’s book is that his explanations are clear to all. It is cram full of essential information for the careful shopper. Every essential term, like preservatives, stabilizers, emulsifiers, tenderizers and flavouring, is clearly spelt out.
This book is not just for those who did not buy the first edition. It contains so much that parents need to know. Do additives affect ability, and what about hyperactivity in children? The fact is that more and more is now known about the effects of what we eat—and we all need to know.
We know that there is a close link between what we eat and our physical—and maybe our mental—health. I doubt whether anyone else in Britain has done more to cut through the commercials and bring out the facts. Maurice Hanssen has been at the forefront of this food and health revolution. For me, it is a real pleasure to commend this new edition. I hope that it, too, will be a bestseller.
THE RT. HON. LORD ENNALS
HOUSE OF LORDS
Foreword to First Edition
This comprehensive book is one which in a sense I wish need never have been written. I would prefer to live in a world where we harvested our foods fresh from the earth, ate them immediately and never had to give a thought to food preservatives, artificial emulsifiers and stabilizers, anti-oxidants and permitted colours. Alas, we do not live in such a world. High technology food production and elaborate chains of food distribution have created a situation in which food additives are necessary. Yet for the protection of oneself and one’s family it is also necessary to be well informed about these hundreds of additives in quite specific terms and highly aware of the possible implications of their inclusion in our daily diet.
I therefore welcome Maurice Hanssen’s E for Additives. Mr Hanssen has produced a simple-to-follow yet remarkably ambitious guide which can help people make informed decisions about the foods on their supermarket shelves even before they buy them. He carefully explains both the pros and cons of food additives, clarifies the meaning of such commonly used but little understood words as ‘stabilizers’ and ‘tenderizers’, and offers a quick-to-use guide to each specific additive, its name, where it comes from, the possible adverse effects of using it, and a list of typical products in which it is used. This book is a useful tool for anyone concerned about the health of himself and his family. I for one would not want to be without it.
LESLIE KENTON
Introduction
My first encounter with food additives was in the 1950s when I was concerned with creating new products for people on special diets. It soon became clear to me that many food technologists were using a wide variety of additives simply because they were available, and that they had not really given any thought to the nutritional or health consequences of what they were doing.
I asked the question: ‘Why are we using ingredients that I would not need in the kitchen when preparing the same food?’ Sometimes there were good technical reasons, but in 90 per cent of the cases there was none. It is because I enjoy cooking at home and because I have a strong background in practical food technology on a factory scale that I began to question whether or not we had true freedom of choice whether we knew what we were eating and whether many of the additives were necessary at all.
In the 1960s, with the National Association for Health, sponsored by Joyce Butler MP, and with the help of 750,000 well-wishers, we presented a petition to Parliament asking them to ‘add all additives’. This was a plea to have a full label declaration of all the ingredients.
For the past 100 or so years there has been an artificial division in our minds between foods and medicines. Since the earliest times man has known that he can live on a wide variety of foods, and that some apparently attractive plants are dangerous whilst some help bring vibrant health and fitness. Even more sophisticated has been the use of very small quantities of otherwise dangerous herbs, such as foxglove or deadly nightshade, which are both still today very important medicines in minute doses.
To stay at the peak of fitness a Roman soldier was only allowed stoneground wholemeal flour. None of the sifted white flour, beloved of the rulers of Rome, found its way into his diet. In the Middle Ages, writers on health said that ‘the bread which had all the bran in it was a remedy for constipation caused by eating too much of the fine white bread’! It is obvious that the foods we eat are more important than any additives. But in general terms we have had personal control over our choice of food but little influence on the additives being used.
The 1984 Food Labelling Regulations gave us, for the first time, a good insight into what we were eating and gave me the chance to write E for Additives. Even if the book had not sold a single copy I would have needed it for myself and my family. But in the event it was a bestseller which has prompted fundamental changes in the food that we buy. Almost overnight, crisp manufacturers found that they could remove E320 and E321. This may have reduced the shelf-life of the crisps but, with the odd exception of Scotland where apparently food takes a long time to be delivered, the additive-free crisps lasted quite long enough for any shop with a good turnover of stock. This story was repeated, with a wide range of unnecessary and, to some sensitive people, harmful additives, being removed.
A close and careful reading of The New E for Additives will show you that there are doubts about only 1 in 5 of the additives commonly used in British food. Some of these have been the most common but, fortunately, public pressure is reducing their usage. Toxicity is dose related and at some level of intake all foods are toxic We have to keep a balance, but we also have to ensure that we are not being misled with our senses distorted by the use of additives so that high fat and high sugar foods with a very low essential nutrient content give the feeling, appearance, and taste that they are good balanced nutrition. They may be, but an informed look at the label can in most cases give the true picture.
E for Additives provoked a huge correspondence from both consumers and manufacturers, a lot of which was extremely useful in preparing this new edition. It reflects the vast amount of new knowledge that has become available during the intervening three years and its purpose is to increase understanding and to encourage the enjoyment of good, well-prepared foods whether they be in the home, restaurant, health store, or supermarket.
1. How to Read the Label
Since 1 January 1986 most foods have had to carry a relatively complete list of ingredients. Flavourings do not have to be declared, except by the word ‘flavourings’, but all the other ingredients, including water, have to be listed in descending order by weight, determined as at the time of their use in the preparation of the food. Water, when there is more than 5 per cent, and other volatile products which are added as ingredients of the food, are listed in order of their weight in the finished product, the weight being calculated in the case of water by deducting from the total weight of the finished product the total weight of the other ingredients used.
If an ingredient used in food is in a concentrated or dried form and becomes reconstituted during the preparation of the food then the weight, in determining the order of the list of ingredients, can be the weight of the ingredient before it has been concentrated or dried. If the food is itself a mixture of concentrated or dried ingredients which have to be reconstituted by adding water, then it is allowable to list the ingredients in descending order of their weight when reconstituted provided that, instead of just saying ingredients’, the list is preceded by the words ‘ingredients of the reconstituted product’, or something similar.
If a food consists of, or contains, mixed fruits, nuts, vegetables, spices, or herbs and no particular fruit, nut, vegetable, spice, or herb predominates significantly by weight, the ingredients can be listed in no particular order if the list is headed by a phrase such as ‘in variable proportion’, and if the variable proportion mix is just a part of the list of ingredients, then the producer can state that that part of the ingredients list is in variable proportion.
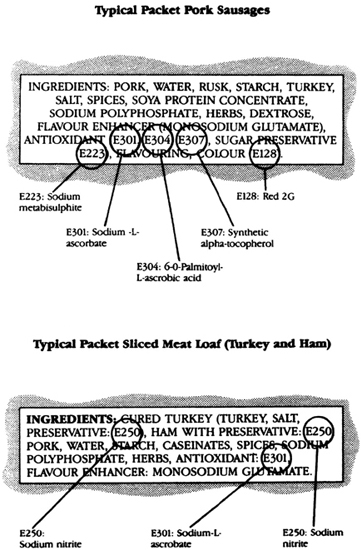
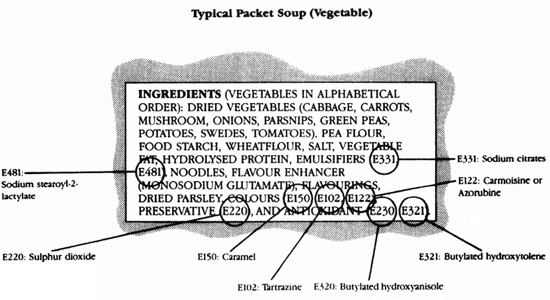
Therefore, with a few exceptions, ingredients are listed in descending order by weight. It is very important to take this into account when reading the label. Many soup or dessert mixes have remarkably similar lists of ingredients in which sugar, starch or flour of some sort, and hydrogenated vegetable fat are high up on the list of ingredients, and sometimes the designated variety of the product such as tomato or strawberry is present in small amounts, or maybe altogether absent.
Food has to be described in a way which is not misleading, using, where there is one, the name prescribed by law, and if there is not, then a customary name, and failing that a precise enough description to inform the purchaser of its true nature and, if needed, a description of its use A made-up name cannot be used instead of the proper name of the food.
‘Flavour’ is a word that does not mean quite what it seems to because if a product is, for example, ‘strawberry flavour’ then it need not contain any strawberry at all. If it is ‘strawberry flavoured’ then a significant part of its flavour must be from strawberries, and if it is ‘strawberry’ then it is made with whole strawberries. This is a rule of thumb which is not enshrined in law, and a number of manufacturers and Local Authorities are of the view that both words ‘flavour’ and ‘flavoured’ are themselves misleading, and a proper description of the product which does not contain any of the designated substance would be ‘artificially flavoured’. Until this is tested in the High Court, or a new regulation is made, the consumer is left with an uncertain and misleading situation.
‘No added sugar’ is another area of potential misinformation. Because many people are worried that too much sugar will cause them to put on excess weight they look out for products which are sugar-free or contain no added sugar. This description is applied even when the food contains a very large quantity of naturally occurring sugars. An example is jam made without added sugar but with concentrated apple or pear juice containing a naturally high level of sugar. Sugar is being interpreted by certain manufacturers as being just the use of sucrose (table sugar). Other sugars, such as lactose and fructose, are sometimes also included in products which are said to have no added sugar.
Certain diet products are equally misleading; for example, there is a diet bar on sale which has sugar as its second largest ingredient. There is also a tendency for manufacturers to say ‘no added colour’ or ‘no preservatives’ or ‘no artificial ingredients’, all of which may be true but does not alter the fact that the food itself is of low nutritional worth. There is no substitute for reading the ingredient list.
Date Marking
Date marking is now required on most pre-packed foods (with a few exceptions, such as frozen foods, wine and vinegar) unless they have a shelf-life of at least 18 months. Even products with a very long shelf-life may be marked, but this is not mandatory. This is expressed as either:
• A best before date (day, month, year) plus storage conditions (if necessary).
Or:
• If the food has a ‘life’ of between 3 months and 19 months, a best before end date (month, year).
• If the food has a ‘life’ of between 6 weeks and 3 months, a best before date (day, month) plus storage conditions (if necessary).
• If the food is perishable and is intended for consumption within 6 weeks of being packed, a sell by date (day, month) plus storage conditions and a storage period after purchase.
There is no reason why you should not buy overdue products, especially if they are reduced in price, because the onus is on the shopkeeper to provide goods which live up to the quality of their description, in other words they must not be bad or ‘off’. With the longer time datings you are safe in buying goods that are near the end of their expiry date if the shop is clean and well maintained. However if such a product has deteriorated, even if bought at a special price, your legal rights are not affected and you should complain first of all to the shop manager then, if no satisfaction is obtained, to your local Trading Standards Officer, whom you can locate through the Town Hall. It is often preferable, though, to write a nice letter, fully documented, with a sample, to the Managing Director of the company concerned who will often, for the sake of goodwill (and most of the food companies are very jealous of their good reputation), refund your cost and may even give you something extra besides. However, if you are on the make, beware, because most manufacturers keep very accurate records of complainants and get wise to the person who frequently finds a dead mouse in a meat pie.
Foods for special nutritional purposes are subject to the provisions of an EEC Directive which strictly controls all claims and declarations in respec of infant, diabetic, slimming and other foods which purport to be for a group of people with special nutritional needs. There is a problem in that some excellent foods which have a nutritional purpose may not, in the future, be able to declare it without a Medicines Licence! For example, a bran based breakfast cereal may not be able to say that it ‘helps constipation’, but it can say ‘helps to keep you regular’ as that is not a medical claim. Too often we are seeing legislation which is designed for consumer protection which effectively shields the consumer from the information needed to make an informed decision. It should surely be sufficient with regard to most claims that labels and advertising are decent, honest and truthful.
Polyunsaturated Fatty Acid Claims
The COMA and NACNE reports on what we should eat for a healthy diet include in their recommendations the view that we should cut down on our total fat intake, have a relatively high proportion of polyunsaturated fatty acids (which are the sort of fats that you have in oils like sunflower, safflower, soya and corn) and consume less animal and dairy fat. This is because such a dietary change is thought to be good for the heart. However, the manufacturer is not allowed by law to tell you that! Before any claims relating to polyunsaturated fatty acids can be made the food has to contain at least 35 per cent of fat by weight. In that fat at least 45 per cent of the fatty acids must be polyunsaturated and not more than 25 per cent saturated.
The claim has to be accompanied by the words ‘low in saturates’ or ‘low in saturated fatty acids’ and the food must be marked with a declaration in grammes per 100 grammes or millilitres of the food stating the amount of fat or oil and the amount of polyunsaturated fatty acids (which are cis, cis-methylene interrupted polyunsaturated fatty acids) and also the amount of saturated fatty acids. Each pan of the declaration has to be given equal prominence.
If, in addition, the claim is made that it is low in cholesterol, then the food must not contain more than 0.005 per cent of cholesterol and it must be possible to make polyunsaturated fatty acid claims. As in the former case there can be no expressed or implied suggestion that such products are beneficial to health. You have to read the label carefully to see that such a claim is being made if you want to choose truly polyunsaturated margarines such as Flora or, from health stores, the very desirable Vitaquell which contains no animal or dairy ingredients and which has not been hardened by the hydrogenation process.
In the USA sensible and accurate claims for reduced cholesterol foods are allowed as are true statements about the advantages of polyunsaturates. So long as such claims are well controlled they could help many people to change their diet for the better and lessen the risk of heart disease.
Vitamins and Minerals



